Alcohols, Epoxides and Ethers
Alcohol Oxidation: “Strong” and “Weak” Oxidants
Last updated: November 10th, 2022 |
Simplifying Alcohol Oxidation: “Strong” Oxidants and “Weak” Oxidants
Here’s what we’ll talk about today: reagents for the oxidation of alcohols.
For the purposes of introductory organic chemistry, it’s helpful to break oxidants for alcohols into two categories: “weak” and “strong“.
- “Weak” oxidants such as pyridinium chlorochromate (PCC), Dess-Martin Periodinane (DMP), and the Swern will only oxidize primary alcohols to aldehydes.
- “Strong” oxidants such as chromic acid (H2CrO4) and KMnO4 will oxidize primary alcohols to carboxylic acids.
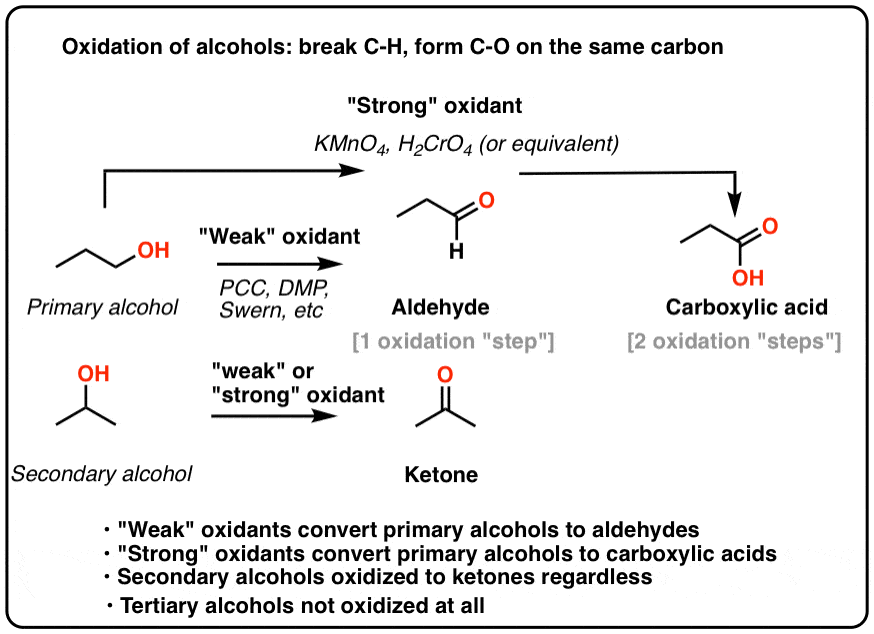
Table of Contents
- An Oxidation Reaction Forms C-O and Breaks C-H On The Same Carbon
- Another Oxidation: Secondary Alcohols To Ketones
- Oxidation of Primary Alcohols To Carboxylic Acids
- Tertiary Alcohols Do Not Undergo Oxidation
- Climbing The Oxidation Ladder, One or Two Rungs At A Time
- “Weak” Oxidants Oxidize Primary Alcohols To Aldehydes – And Stop There
- “Strong” Oxidants Oxidize Primary Alcohols To Carboxylic Acids
- Summary: “Strong” and “Weak” Oxidants for Alcohol Oxidation
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. An Oxidation Reaction Forms C–O and Breaks C–H On the Same Carbon
Here we are, at least fifteen articles into this series on alcohols, and all we’ve really talked about is substitution and elimination reactions, with a little bit of acid-base chemistry mixed in.
We haven’t even scratched the surface of one of the most important classes of reaction for alcohols – one that becomes crucial as you move into Org 2.
I’m talking about oxidation reactions.
What do I mean by an “oxidation reaction”, anyway?
Let’s start by examining the bonds that form and the bonds that break in this process, where we convert a primary alcohol to an aldehyde:
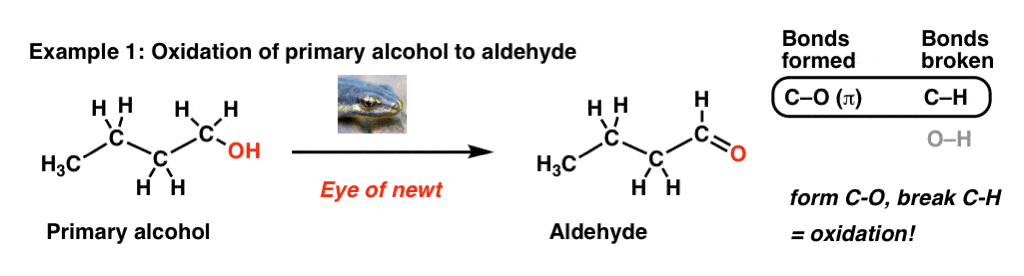
No, “Eye of newt” doesn’t actually do oxidation reactions; a specific example of an oxidant here could be PCC, DMP, or Swern oxidation. The point here is not to focus on the specific reagent but to pay attention to the bonds that form and break in this reaction.
The key process here is that we’re forming a C-O bond and breaking a C-H bond on the same carbon. That’s a sure sign of an oxidation reaction. For more background on oxidation reactions in organic chemistry, check out this previous post [ Quick summary: compare oxidation states in carbon to those in transition metals. Essentially we’re trading a bond between carbon and an atom less electronegative than carbon (H) for one more electronegative than carbon (O). If you think back to Gen Chem and transition metal oxidation states, this would correspond to an increase in oxidation state].
2. Another Oxidation: Secondary Alcohols To Ketones
Here’s another example for you. Starting with a secondary alcohol, we throw in some “wing of bat”, and voila! We end up with a ketone!
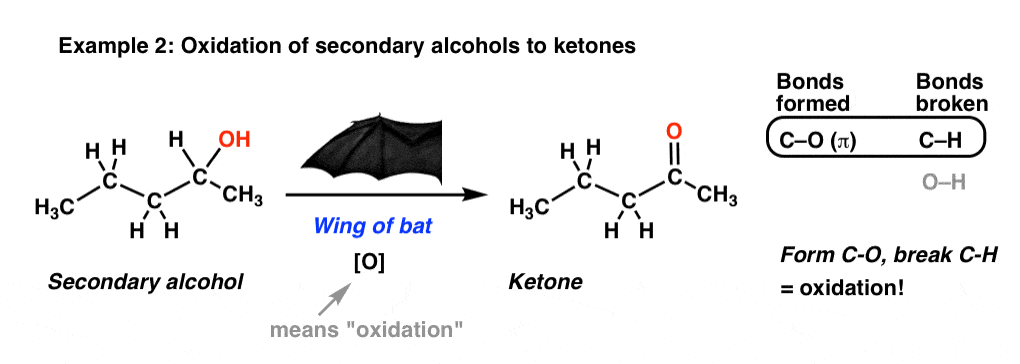
Again, we’re breaking C-H and forming C-O here. By the way, that [O] is shorthand you’ll see occasionally: it just means, “oxidation”.
3. Oxidation of Primary Alcohols To Carboxylic Acids
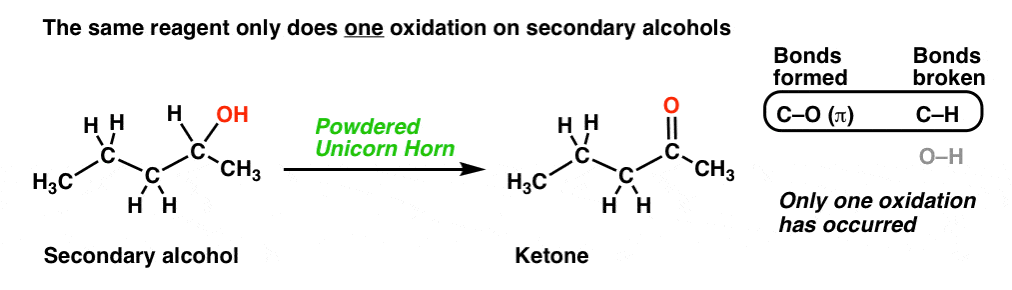
Now let’s look at this third reaction, where we break out some very precious “powdered unicorn horn”.
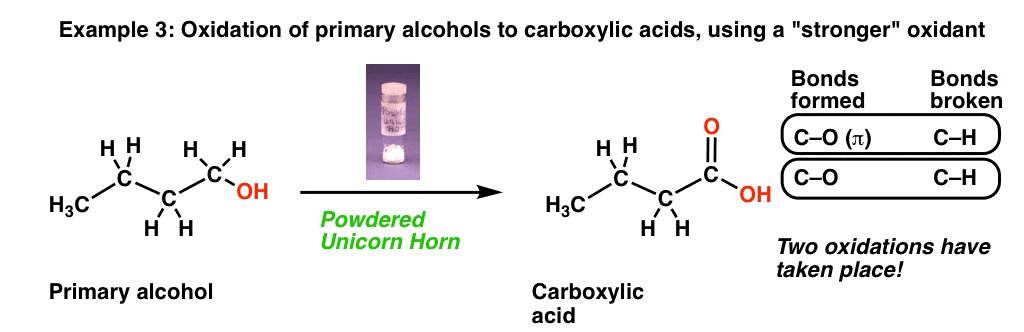
That powdered unicorn horn is powerful stuff! When we count the bonds formed and broken, notice that we formed two new C-O bonds and broke two C-H bonds. In other words, using this one reagent resulted in two oxidations at the same carbon!
Note, however, that our magic unicorn horn only performs one oxidation on secondary alcohols, giving us a ketone (just like, the previously described “wing of bat”)
4. Tertiary Alcohols Do Not Undergo Oxidation
Finally, just so tertiary alcohols aren’t left out, let’s see what happens when they’re treated with any one of our magical reagents.
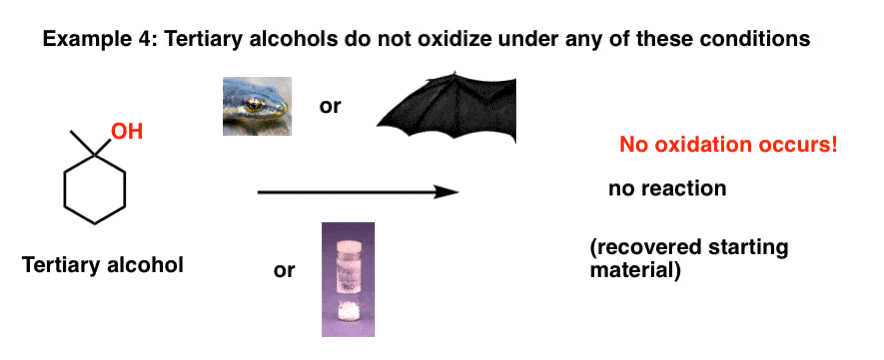
Nothing! Eye of newt, wing of bat, powdered unicorn horn – no matter how powerful, none of them are able to oxidize our tertiary alcohol.
Why is that? If you look closely, there’s no C-H on the carbon of the tertiary alcohol and no oxidation occurs. We would have to break a C-C bond in order for an oxidation to occur, and none of these reagents are competent to do this [for a clue as to why, see the next post]
[BTW: that carbon of an alcohol attached directly to C-OH is sometimes called the “carbinol” carbon]
5. Climbing The Oxidation Ladder, One Or Two Rungs At A Time
Let’s organize all of these reactions into a table. In one of the most powerful analogies in organic chemistry, imagine we have a “ladder” of oxidation states for carbon. Alcohol is at the bottom of our miniature ladder here, and each oxidation goes a step up [we say “oxidize up” because we’re increasing the oxidation state, i.e. making it more positive].
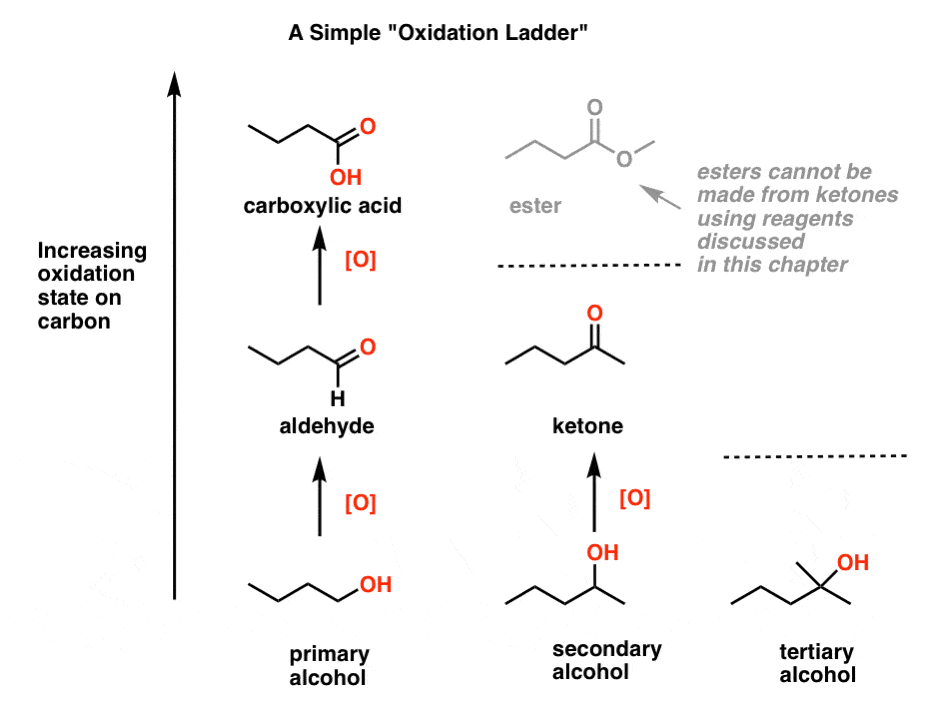
One step “up” from a primary alcohol is an aldehyde, which we showed in example 1.
Two steps “up” from a primary alcohol [and one step up from an aldehyde] is a carboxylic acid, which we showed in example 3.
One step “up” from a secondary alcohol is a ketone, which we showed in example 2 and also with a “stronger” oxidant in example 3.
And in example 4 we showed that oxidation “up” from a tertiary alcohol isn’t possible even with the help of these “magical” reagents we’ve been using.
By the way, shouldn’t there also be a step “up” from a ketone? Yes – it would be an ester [shown in grey]. But none of the reagents we’ll talk about here are capable of climbing this rung of the ladder [again, it would involve breaking a C-C bond]. Later on, you might learn about a special way to do it [See post: Baeyer-Villiger Reaction] which we’ll discuss when we go through reactions of ketones.
6. “Weak” Oxidants Oxidize Primary Alcohols To Aldehydes, and Stop There
Now we’re going to stop being so silly and get to specific reagents instead of talking about “eye of newt”, “wing of bat”, and “powdered unicorn horn”.
But first, here’s a useful distinction to make in keeping track of oxidizing agents.
“Weak” Oxidants
Some oxidants will react with primary alcohols to give aldehydes, and stop there. Let’s call these “weak” oxidants.
They don’t go on to the next “rung” up i.e. carboxylic acids.
It’s a bit of an oversimplification [Note 1] but it will do for our purposes.
Examples of this are pyridinium chlorochromate (PCC), Dess-Martin Periodinane (DMP), the Swern oxidation [(COCl)2, DMSO, NEt3)] and CrO3/pyridine (the “Collins reagent“) all shown below. There are many, many more oxidants that will do this transformation than those I just mentioned; these are merely the reagents encountered most often in undergraduate courses.
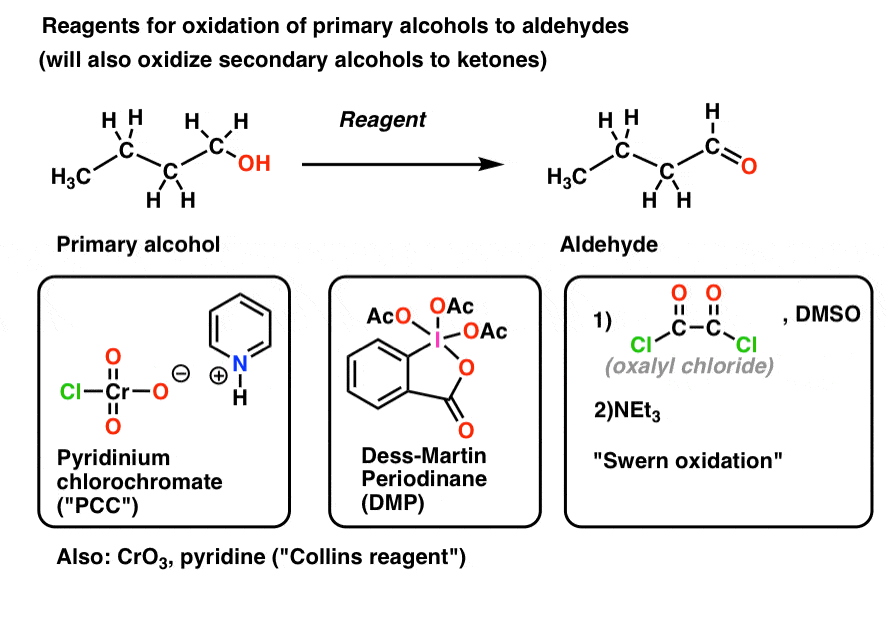
As mentioned, these oxidizing agents will also convert secondary alcohols to ketones.
7. “Strong” Oxidants Oxidize Primary Alcohols To Carboxylic Acids
A second class of oxidants are more vigorous. They will convert primary alcohols to carboxylic acids [two steps “up”] in one flask.
Let’s call these strong oxidants. They fall into two general categories: potassium permanganate (KMnO4) and Cr(VI) species, which are essentially different precursors of chromic acid (H2CrO4).
[I’ve written before that H2CrO4 is one of the most annoying reagents in organic chemistry, because it has so many potential precursors spread throughout different textbooks. K2Cr2O7, Na2Cr2O7, Na2CrO4, K2CrO4, CrO3/H3O+, Jones reagent – they’re all essentially the same thing, at least for our purposes. ]
They will also oxidize secondary alcohols to ketones (and stop there).
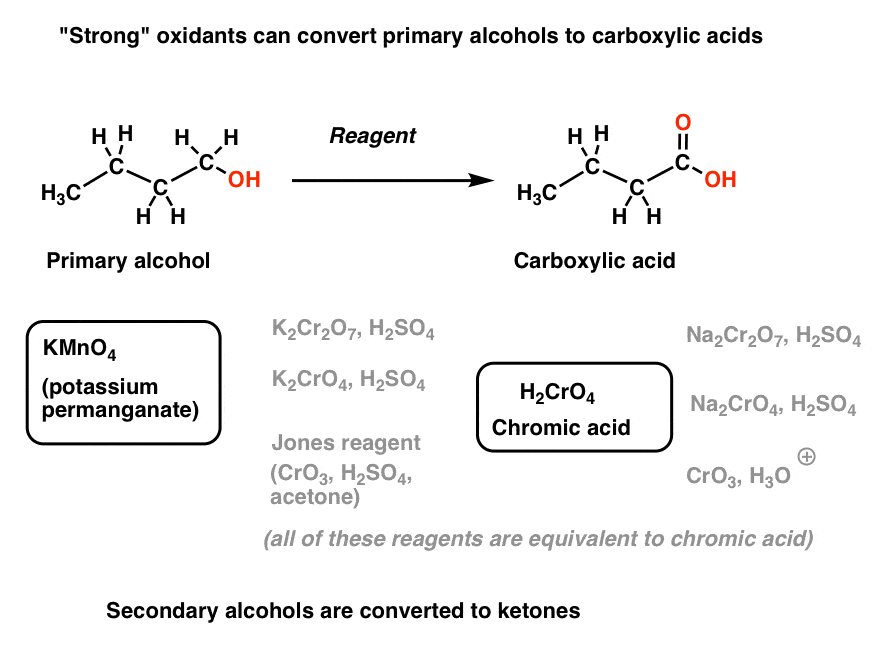
And like I said earlier, neither strong nor weak oxidants will oxidize tertiary alcohols.
8. Summary: Strong and Weak Oxidants for Alcohol Oxidation
Let’s make a table, shall we? This is the “bottom line” for this post.
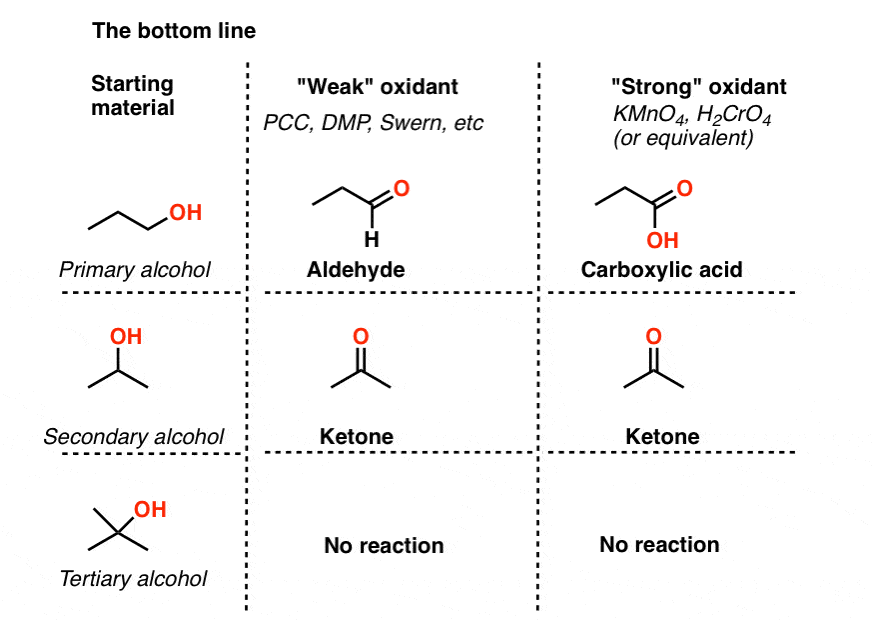
- “Weak” oxidants convert primary alcohols to aldehydes and stop there. They also oxidize secondary alcohols to ketones.
- “Strong” oxidants convert primary alcohols to carboxylic acids. They also oxidize secondary alcohols to ketones.
- None of the reagents we’ve encountered will oxidize tertiary alcohols.
Next time – How Oxidation Reactions Work
So how do these mysterious reagents work, anyway?
Reagents for oxidation of alcohols was one of those things that made me feel really dumb when I learned organic chemistry.
The reagents that were given to us might have well been “eye of newt” and “powdered unicorn horn” since they were introduced without any background or context and disappeared just as quickly after the section on oxidation was over.
It was only later that I understood that oxidation is not nearly as complicated as these weird reagents make it seem. In fact, the underlying process is in most cases extremely familiar – it’s just not taught that way!
In the next post, we’ll discuss the common – and very familiar! – mechanistic step that (almost) all oxidation reactions you’ll learn have in common. Not only will oxidation reactions then become less mysterious.
Next Post – Demystifying The Mechanisms of Alcohol Oxidations
Notes
Note 1. It’s an oversimplification because the first step in oxidation of an aldehyde is generally addition of water to form a hydrate, which is then oxidized to the carboxylic acid. Some oxidants we call “weak” (e.g. CrO3, pyridine) can thus be “strong” if water is present. It’s a teaching kludge, but good enough for our purposes, for now.
Quiz Yourself!
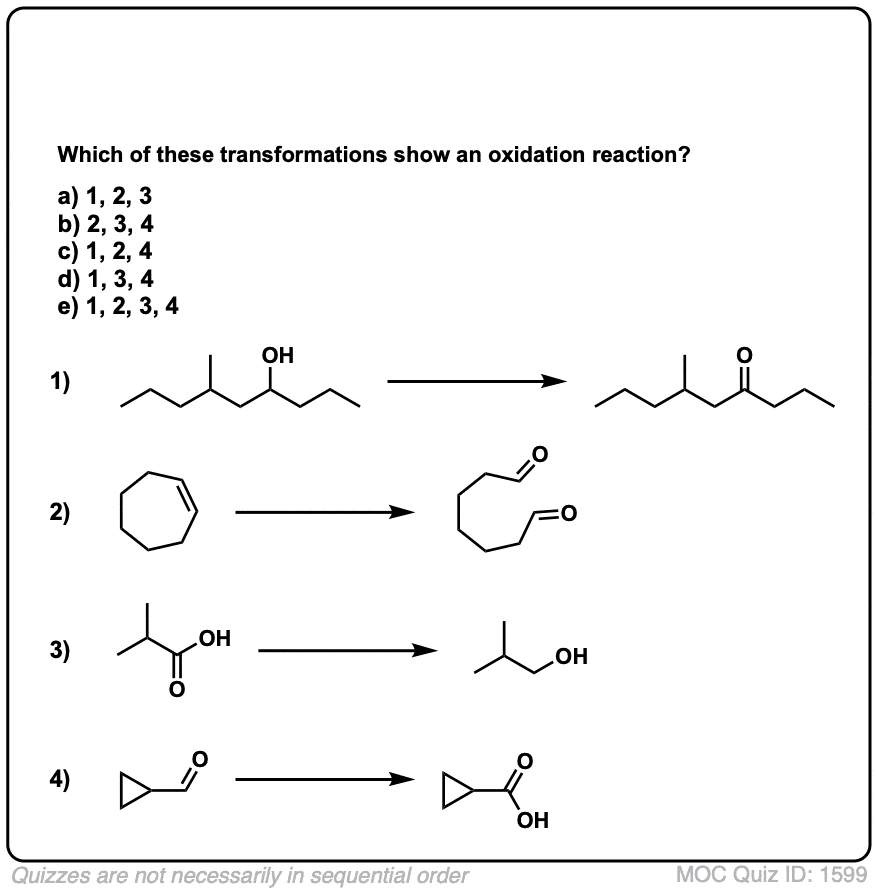 Click to Flip
Click to Flip
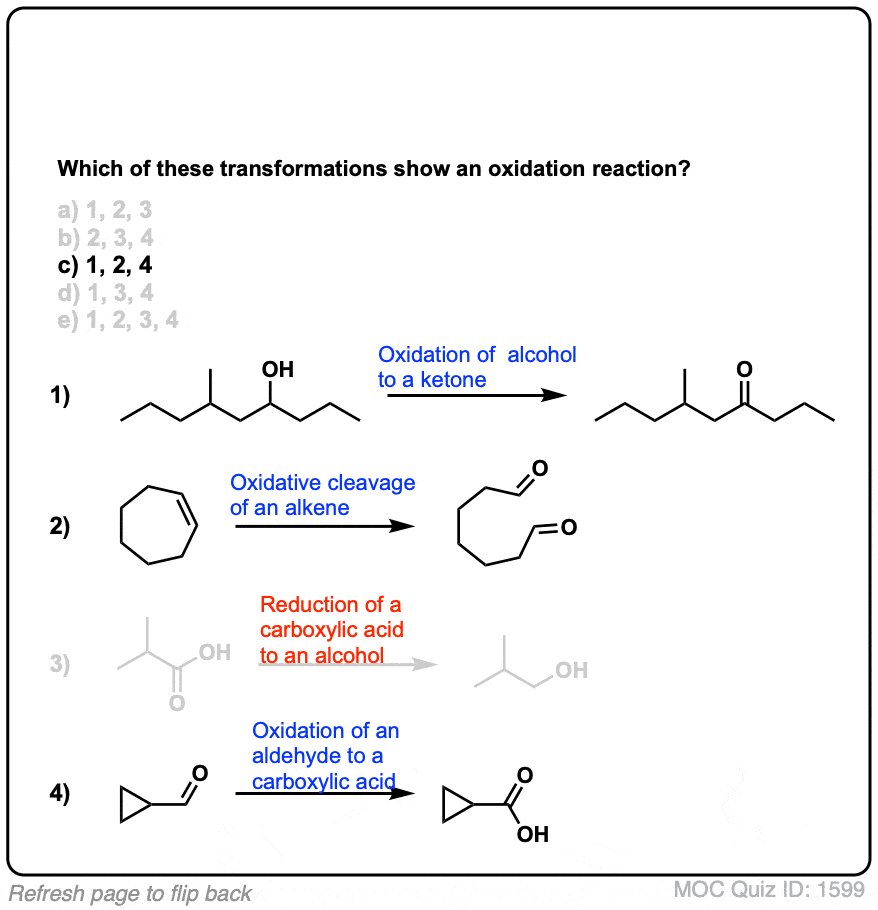
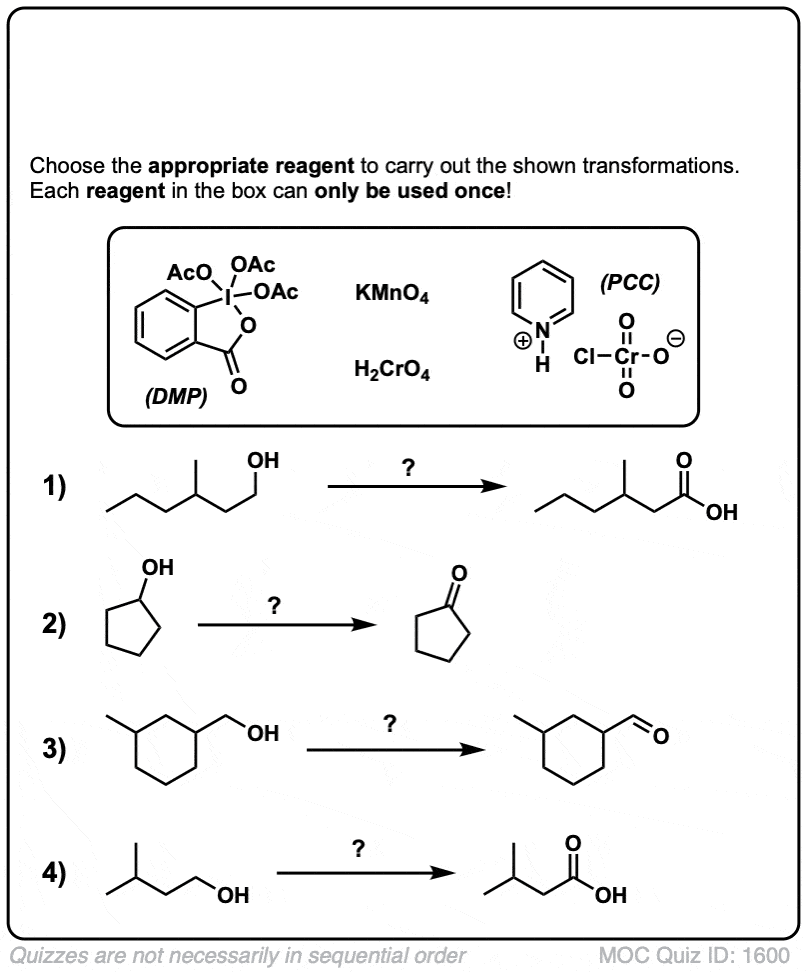 Click to Flip
Click to Flip
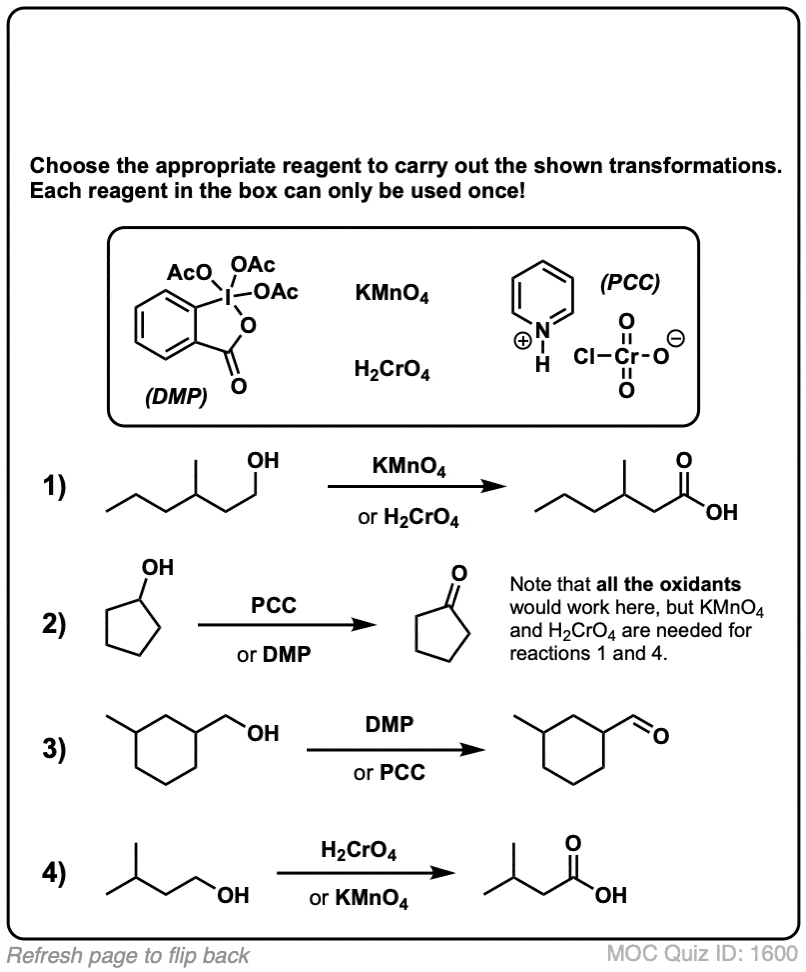
(Advanced) References and Further Reading
Oxidation of secondary alcohols (‘weak’ oxidation):
- Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones
D. B. Dess and J. C. Martin
The Journal of Organic Chemistry 1983, 48 (22), 4155-4156
DOI: 10.1021/jo00170a070 - A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species
Daniel B. Dess and J. C. Martin
Journal of the American Chemical Society 1991, 113 (19), 7277-7287
DOI: 1021/ja00019a027
These two articles are on the development and use of the compound now known as “Dess-Martin periodinane”, a hypervalent I(V) compound that has found widespread use as a mild oxidant in organic synthesis. Prof. J. C. Martin spent most of his career at University of Illinois Urbana-Champaign and ended his career at Vanderbilt University. During his career he contributed a lot towards our understanding of hypervalent main-group chemistry, preparing many S(IV), S(VI), Br(III), I(III), I(V), and I(VII) compounds, among others. - Oxidation of alcohols by “activated” dimethyl sulfoxide. A preparative, steric and mechanistic study
Omura, K.; Swern, D.
Tetrahedron 1978, 34 (11): 1651–1660
DOI: 1016/0040-4020(78)80197-5 - Structure of the dimethyl sulfoxide-oxalyl chloride reaction product. Oxidation of heteroaromatic and diverse alcohols to carbonyl compounds
Mancuso, A. J.; Brownfain, D. S.; Swern, D.
Org. Chem. 1979, 44 (23): 4148–4150
DOI: 10.1021/jo01337a028 - Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide “activated” by oxalyl chloride
Mancuso, A. J.; Huang, S.-L.; Swern, D.
J. Org. Chem. 1978, 43 (12): 2480–2482
DOI: 10.1021/jo00406a041 - Mechanisms of dimethylsulfoxide oxidations
Kurt Torssell
Tetrahedron Letters 1966 7 (37), 4445-4451
DOI: 1016/S0040-4039(00)70057-8
These papers are on what is now commonly called the “Swern oxidation” after its developer, Daniel Swern. This method is rather mild and uses DMSO, a common solvent, as the oxidant. However, this also results in the formation of dimethyl sulfide (which is notoriously stinky) as the product of the reaction, one of its noteworthy characteristics. - SYNTHESIS OF 1,1-DIMETHYLETHYL (S)-4-FORMYL-2,2-DIMETHYL-3-OXAZOLIDINECARBOXYLATE BY OXIDATION OF THE ALCOHOL
Alessandro Dondoni and Daniela Perrone
Org. Synth. 2000, 77, 64
DOI: 10.15227/orgsyn.077.0064
The final step (4 -> 5) in this multistep procedure is a Swern oxidation. This is from Organic Syntheses, a source of reliable, independently tested synthetic organic procedures. - New and highly effective method for the oxidation of primary and secondary alcohols to carbonyl compounds
E. J. Corey; C. U. Kim
Journal of the American Chemical Society 1972, 94 (21): 7586–7587
DOI:10.1021/ja00776a056. - A method for the oxidation of sec,tert-1,2-diols to α-hydroxy ketones without carbon-carbon cleavage
E. J. Corey; C. U. Kim
Tetrahedron Letters 1974, 15 (3): 287–290
DOI:10.1016/S0040-4039(01)82195-X
These papers by Nobel Laureate Prof. E. J. Corey (Harvard) are on the development of what is now known as the “Corey-Kim” oxidation. This is very similar to the Swern oxidation in that DMSO is used as the oxidant, except that here NCS (N-chlorosuccinimide) is used instead of oxalyl chloride. The advantage with this procedure is that temperatures above –25 °C can be used, and the disadvantage is that substrates susceptible to chlorination by NCS cannot be used. - A New and Selective Oxidation of Alcohols
Pfitzner, K. E.; Moffatt, J. G.
J. Am. Chem. Soc. 1963, 85: 3027
DOI: 10.1021/ja00902a036
This paper describes yet another DMSO-based oxidation, the Pfitzner-Moffatt oxidation, which is not used that much anymore due to the difficulties in separating out the dicyclohexylurea byproduct from the desired product. - EINE METHODE DER DEHYDRIERUNG VON SEKUNDAREN ALKOHOLEN ZU KETONEN. I, ZUR HERSTELLUNG VON STERINKETONEN UND SEXUALHORMONEN
R. V. Oppenauer
Recl. Trav. Chim. Pays-Bas 56 (2): 137–144
DOI: 10.1002/recl.19370560206
This paper lays the basis for what is now called the Oppenauer oxidation, the oxidation of secondary alcohols to ketones using Al(i-PrO)3 in excess acetone. - Oxidations with Manganese Dioxide
E. P. Papadopoulos, A. Jarrar, and C. H. Issidorides
The Journal of Organic Chemistry 1966, 31 (2), 615-616
DOI: 10.1021/jo01340a520
As this paper shows, MnO2 can also be used for oxidation of secondary alcohols.Oxidation with PCC (pydinium chlorochromate): - Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds
E. J. Corey, J. William Suggs
Tetrahedron Letters Volume 16, Issue 31, 1975, Pages 2647-2650
DOI: 10.1016/S0040-4039(00)75204-X
The original paper by Nobel Laureate Prof. E. J. Corey on the use of pyridinium chlorochromate as a mild oxidation reagent in organic synthesis. - The story of the discovery of PCC was rather serendipitous, as explained by Prof. Suggs in this blog post:
Suggs - Pyridinium Chlorochromate: A Versatile Oxidant in Organic Synthesis
Piancatelli, A. Scettri, M. D’Auria
Synthesis 1982; 1982(4): 245-258
DOI: 10.1055/s-1982-29766
Review on the applications of PCC in organic synthesis. Includes a discussion on the mechanism. - Kinetics and Mechanism of the Oxidation of Alcohols by Pyridinium Chlorochromate
Banerji Kalyan K.
Chem. Soc. Jpn. 1978, 51 (9), 2732
DOI: 10.1246/bcsj.51.2732
A nice mechanistic study of PCC oxidation, and includes a probable mechanism of the reaction. - Stoichiometry of the oxidation of primary alcohols with pyridinium chlorochromate. Evidence for a two-electron change
Herbert C. Brown, C. Gundu Rao, and Surendra U. Kulkarni
The Journal of Organic Chemistry 1979 44 (15), 2809-2810
DOI: 1021/jo01329a051
In this paper, Nobel Laureate H. C. Brown proves that PCC oxidations involve a transfer of 2 electrons from the Cr to the substrate. Therefore, one does not need to use an excess of PCC – 1 equivalent works fine. - SYNTHESIS OF 1,2:4,5-DI-O-ISOPROPYLIDENE-D-erythro-2,3-HEXODIULO-2,6-PYRANOSE. A HIGHLY ENANTIOSELECTIVE KETONE CATALYST FOR EPOXIDATION
Yong Tu, Michael Frohn, Zhi-Xian Wang, and Yian Shi
Org. Synth. 2003 80, 1
DOI: 10.15227/orgsyn.080.0001
This tested procedure from Organic Syntheses uses PCC to make the chiral ketone catalyst for asymmetric epoxidation, known as ‘Shi epoxidation’ after its creator, Prof. Yian Shi (Colorado State).Oxidation of primary alcohols to carboxylic acids (‘strong’ oxidation): - Synthesis of a model depsipeptide segment of Luzopeptins (BBM 928), potent antitumor and antiretroviral antibiotics
Marco A. Ciufolini and Shankar Swaminathan
Tetrahedron Letters Volume 30, Issue 23, 1989, Pages 3027-3028
DOI: 1016/S0040-4039(00)99393-6
Step f in the synthesis (Scheme 1) is an oxidation of a primary alcohol to carboxylic acid using KMnO4. - Stereocontrolled addition to a penaldic acid equivalent: an asymmetric of -β-hydroxy-L-glutamic acid
Philip Garner
Tetrahedron Letters Volume 25, Issue 51, 1984, 5855-5858
DOI: 10.1016/S0040-4039(01)81703-2
The final step (g, 6 -> 7) in the synthesis in this paper is an oxidation of a primary alcohol to a carboxylic acid using KMnO4.The Jones oxidation, which uses chromic acid (CrO3 in H2SO4) is a common method for the oxidation of primary alcohols to carboxylic acids. The drawback is of course the production of stoichiometric amounts of chromium waste. - Researches on acetylenic compounds. Part XIV. A study of the reactions of the readily available ethynyl-ethylenic alchohol, pent-2-en-4-yn-1-ol
Sir Ian Heilbron, E. R. H. Jones and F. Sondheimer
J. Chem. Soc., 1947, 1586-1590
DOI: 10.1039/JR9470001586 - An Improved Procedure for the Oxidation of Alkynols to Alkynoic Acids
B. C. Holland and N. W. Gilman
Synth. Commun. 1974, 4, 203-210
DOI: 10.1080/00397917408062073 - Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media
E. J. Corey, Greg Schmidt
Tetrahedron Letters Volume 20, Issue 5, 1979, 399-402
DOI: 10.1016/S0040-4039(01)93515-4
Nobel Laureate Prof. E. J. Corey (Harvard) shows that PDC (pyridinium dichromate) in DMF can be used for the oxidation of primary alcohols to carboxylic acids. - Applications of the peracid-mediated oxidation of alcohols
James A. Cella, James P. McGrath, James A. Kelley, Omaya El Soukkary, and Lawrence Hilpert
The Journal of Organic Chemistry 1977, 42 (12), 2077-2080
DOI: 10.1021/jo00432a008
This paper demonstrates that a peracid (mCPBA) can directly oxidize secondary alcohols to esters, a tandem oxidation-Baeyer-Villiger reaction.
Is there any benefit in using a strong oxidant over a weak one when converting a secondary alcohol to a ketone? TA’s answer key had PCC crossed out in favor of KMnO4
PCC is totally fine.
No reason to use KMnO4 – it is very unselective and will oxidize everything.
what about allylic alchol oxidation
The usual procedure is to use MnO2, although there are other ways. It’s not often covered in North American introductory courses, so I don’t discuss it.
It is very helpful.Thank u so much for helping us…..❤
thank you,this info really helped me
Glad to hear it, thank you aditya.
Don’t tertiary alcohols give alkenes on strong oxidation?
No, and that wouldn’t be an oxidation anyway. That’s elimination.