Addition of Grignard reagents to nitriles to give ketones (after hydrolysis)
Description: Grignard reagents will add once to nitriles to form imines. The imines can be treated with aqueous acid to give ketones.
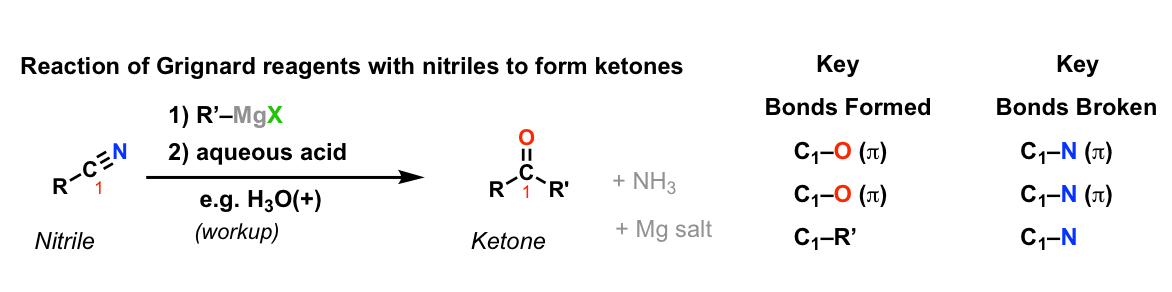
Notes:
- The purpose of the second step is to convert the intermediate imine into a ketone.
- Water (H2O) is generally sufficient although aqueous acid (H3O+) is more effective.
- X here can be Cl, Br or I, depending on how the Grignard reagent is made.
Examples:
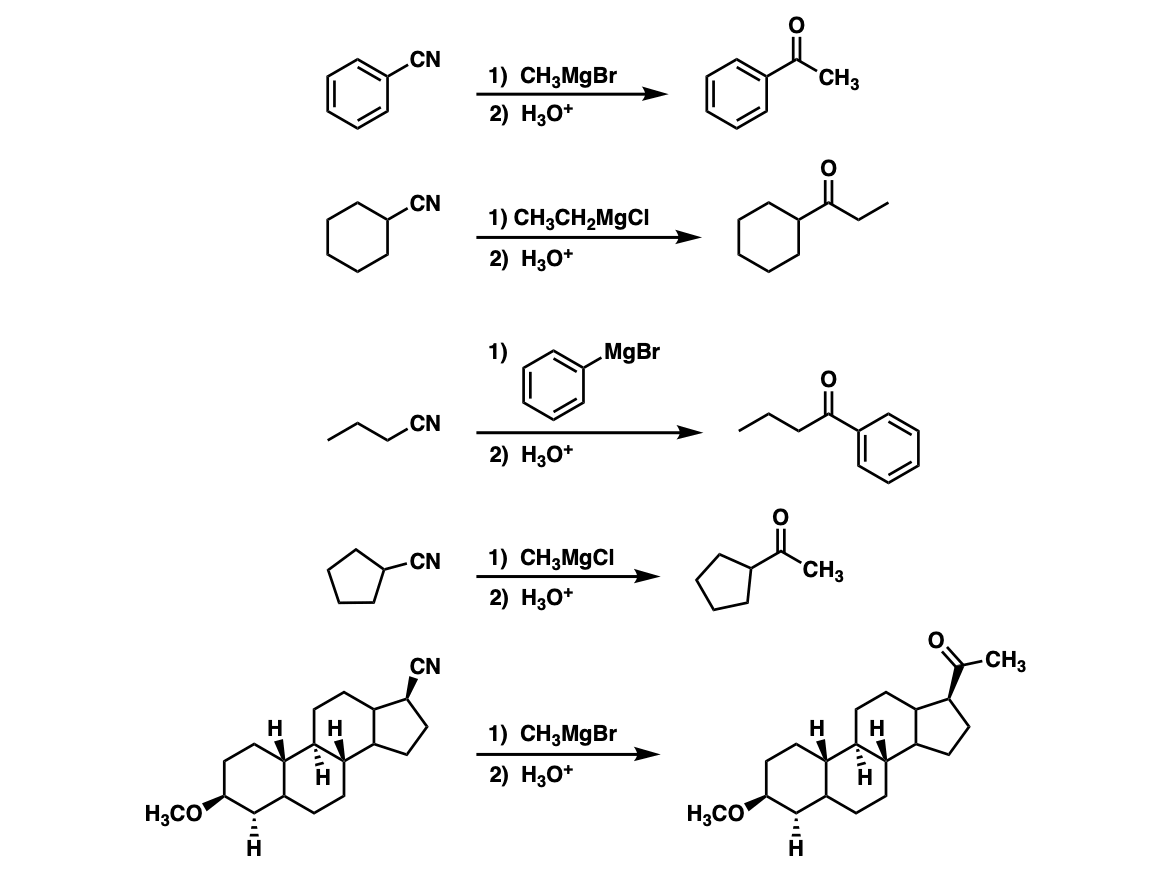
Notes: The first product of the reaction is an imine, which is then hydrolyzed to the ketone with water (or mild aqueous acid). Note the last (5th) example – don’t get distracted by the huge molecule, just pay attention to the nitrile!
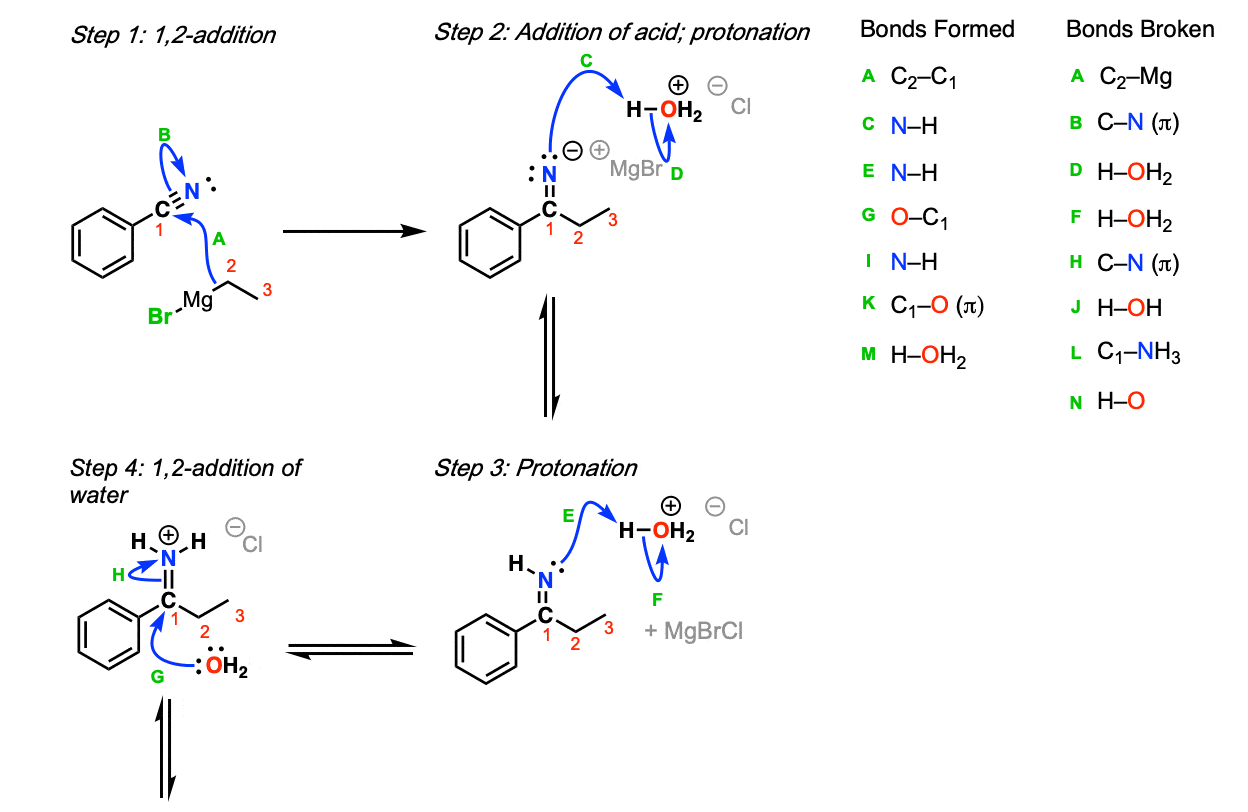
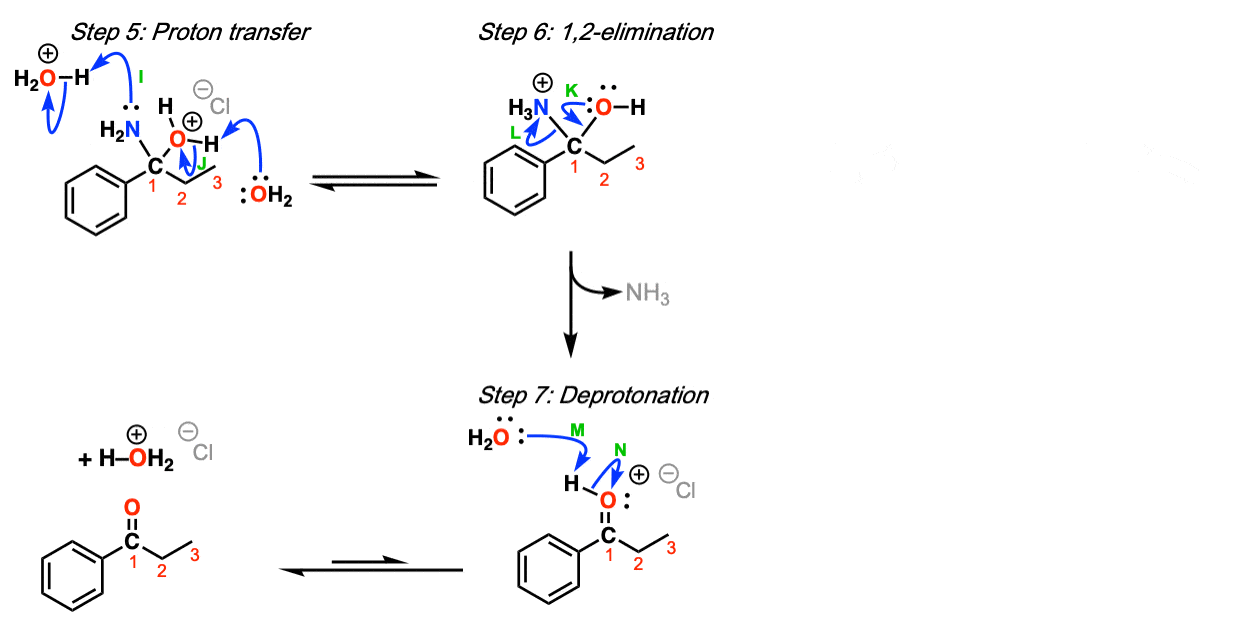
Mechanism:
The Grignard reagent adds to the carbon of the nitrile, forming a new carbon-carbon bond (Step 1, arrows A and B). This is stable until water and acid is added (Step 2, arrows C an D) which forms the imine. Protonation of the imine nitrogen (Step 3, arrows E and F) results in the formation of the iminium ion, which undergoes 1,2-addition by water (Step 4,
arrows G and H). This species then undergoes proton transfer (Step 5, arrows I and J) to allow for the loss of ammonia (NH3) in a subsequent 1,2-elimination (Step 6, arrows K and L). Deprotonation of the carbonyl oxygen with base (Step 7, arrows M and N) then results in the ketone.
Notes:
- Step 2 occurs only after the addition of aqueous acid. Note that a full equivalent of acid is necessary here to protonate the imine.
- Other acids can be used: the use of Cl(-) as a counter-ion for H3O(+) here is arbitrary here and of no consequence in the reaction except to balance the charge from H3O(+).
- Steps 3-7 are identical to those for the hydrolysis of imines.
- Other reasonable mechanisms can be drawn for proton transfer.
- Other bases could reasonably be used instead of water.
- A protonated imine is called an “iminium”.
Quiz Yourself!
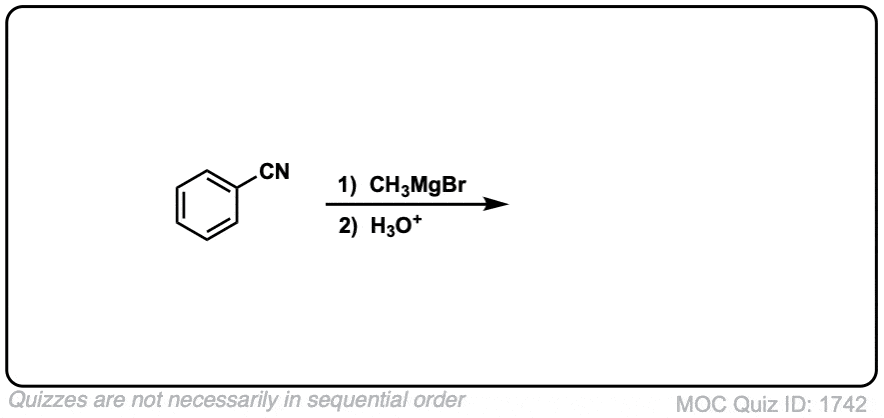 Click to Flip
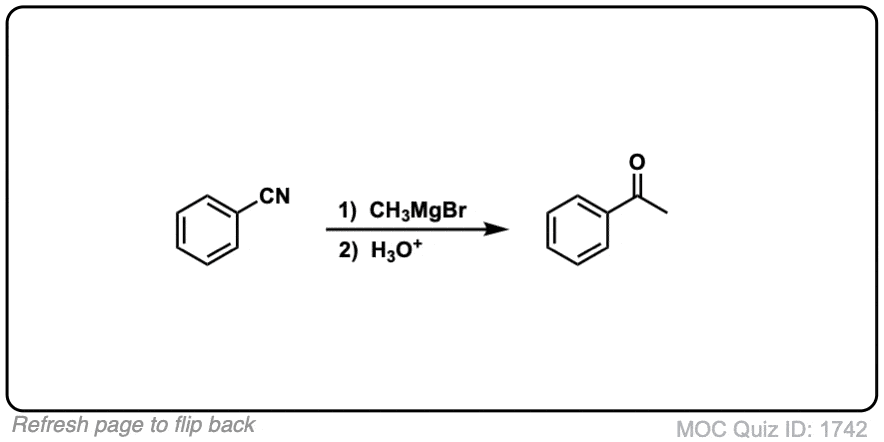
Click to Flip
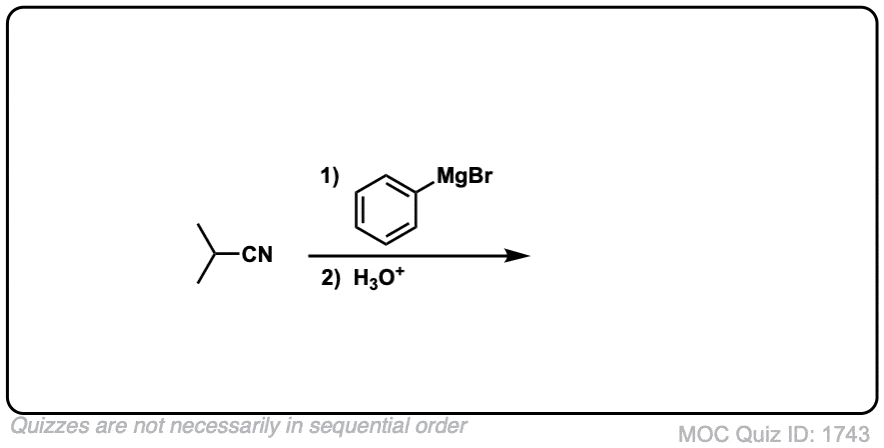 Click to Flip
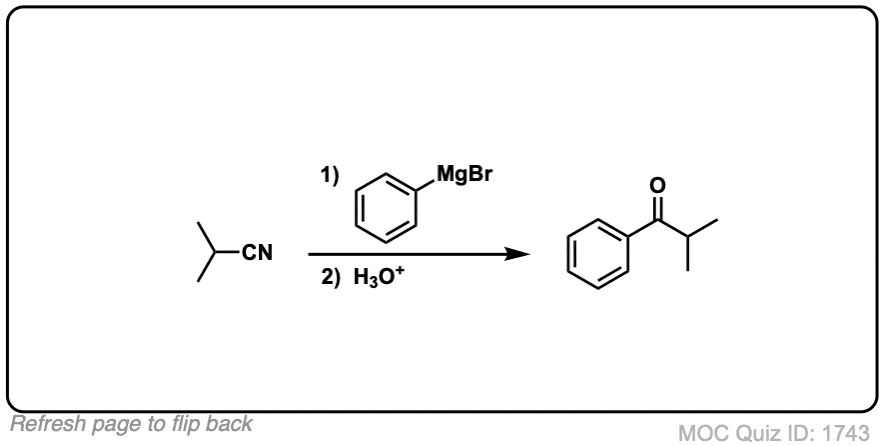
Click to Flip
(Advanced) References And Further Reading
- First example
Kharasch, M. S.; Reinmuth, O. Grignard Reactions of Nonmetallic Substances, Prentice-Hall, Englewood Cliffs, NJ, 1954, pp. 767-845 - Effet du benzene dans la reaction de Grignard sur les nitriles
Persephone Canonne, George B.Foscolos, Gilles Lema
Tetrahedron Letters 1980, 155
DOI: 10.1016/S0040-4039(00)71400-6
Use of benzene containing one equivalent of ether as solvent in Grignard reactions of nitriles at room temperature leads to increased yields of the corresponding ketones compared to results obtained for the same reactions in ether. - Organometallic reaction mechanisms. XII. Mechanism of methylmagnesium bromide addition to benzonitrile
E. C. Ashby, Li-Chung. Chao, and H. M. Neumann
Journal of the American Chemical Society 1973 95 (15), 4896-4904
DOI: 10.1021/ja00796a022
The kinetic data of the title reaction show a second-order reaction, first order in Grignard reagent and first order in nitrile. The results of rate studies in the presence of added MgBr2 show that the reaction of the Grignard reagent with benzonitrile occurs through both the (CH3)2Mg and CH3MgBr species. - The Mechanism of Addition of Grignard Reagents to Nitriles
C. Gardner Swain
Journal of The American Chemical Society, 1947, 69(10), 2306
DOI: 10.1021/ja01202a018
This paper is an early literature reference to this reaction and describes a kinetic study of the reaction between n-butylmagnesium bromide and benzonitrile, which was found to be 2nd order. - Copper(I)-activated addition of Grignard reagents to nitriles. Synthesis of ketimines, ketones, and amines
Franz J. Weiberth and Stan S. Hall
The Journal of Organic Chemistry 1987 52 (17), 3901-390
DOI: 10.1021/jo00226a033
The nucleophilic addition of Grignard reagents to nitriles, especially when using sterically demanding components, is effectively catalyzed by copper(I) salts. - Double Addition of Organometallics to Nitriles: Toward an Access to Tertiary Carbinamines.
Advanced Synthesis & Catalysis, 359(2), 179–201
Pearson-Long, M. S. M., Boeda, F., & Bertus, P. (2016).
DOI: 10.1002/adsc.201600727
This review describes a variant of this reaction – the double addition of organometallics (e.g. Grignard reagents or organolithium reagents) to nitriles.
Did we get one carbon atom or the grignard was Ch3Ch2MgBr
What image does this refer to? The extra carbon comes from the nitrile CN. I don’t see a typo, but if there is a typo please let me know.
For the quiz section, shouldn’t the first question yield a benzene on one side while a methyl on the other side of the carbonyl carbon not an ethyl?
Fixed. Thank you
Just wondering if there is an explanation on why turbo Grignard didn’t attack the nitrile even at 0 degree in Paul Knochel’s work, maybe more complicated mechanistically, but I don’t think I have a good rationale right now. Any thoughts?
I was wondering if the same logic would apply to the Bartoli’s chemistry, that turbo will also leave the nitro untouched…
Can I assume you mean t-Bu Grignard? (Autocorrect for chemistry is a pain).
Not sure. Addition to nitriles is the slowest of all the carboxylic acid derivatives and addition to benzonitrile with n-BuMgBr does occur at RT and at 0. See: https://pubs.acs.org/doi/pdf/10.1021/ja01202a018
I do wonder about what the Schlenk equilibrium looks like for t-BuMgBr though. Do you have a lit reference for Knochel’s stuff?
Not sure about Bartoli but saying as it’s usually run < 0° that would make nitro more reactive than nitriles.
How will you prepare ethane nitrile from grignard reagent
Usually the reaction goes the other direction; reaction of grignards with nitriles.
Ive been conducting this kind of synthesis for a while.I just cant repeat the ketamine synthesis from Zealot.The only difference is that I bought Cyclopentyl magnesium bromide 1mol/L in THF instead of synthesizing myself.The gingnard reagent react with O-chlorobenzonitrile.
Ive tried several times but too many spots shows in TLC.
CuBr added as catalyst but only made the system more complex with littile product.
I wonder the meaning of adding ammonia when hydrolyse the reaction with NH4Cl.I hydrolysed my reaction with both NH4Cl and HCL.The former didnt show any product, but the second one shpowed many spots in TLC.
What is the product formed when hcn reacts with grignard reagent?
HCN is an acid. What do you get when you treat Grignards with acid?
Where did that oxygen come from?
The oxygen comes from adding water in the workup step, which hydrolyzes the imine to the ketone.
When adding the nitrile to the grignard, should it be dissolved in benzene and added by dripping at an appropriate rate to keep things under control?
In general ethereal solvents tend to be used for Grignard reactions (e.g. THF, diethyl ether) but according to March’s Advanced Organic Chemistry 5th Ed., p. 1216, using benzene as a co-solvent increases yields. In one prep I found the Grignard is made and then the nitrile is added quickly at 0°C and then stirred for 3h. Interestingly reactions with nitriles are fairly slow (compared to near-instantaneous for ketones and aldehydes). Double addition is rare. See this prep. http://www.orgsyn.org/Result.aspx?ga=na [Org Syn Coll. 3, p. 26]
If I would use HCN then RCHO would form ?
No, the H would react with the Grignard and it would be destroyed.
On reaction with terminal alkyne Grignard reagent forms alkane but on reaction with hydrogen cyanide it does not form alkane…. Why???
I thought N don’t usually leave?
If it’s protonated, it will.
Is it a Sn1 or Sn2 reaction ?
Neither. Nucleophilic addition (1,2 addition)
Is that possible that alkyl cynide react with two moles of grignard reagent to give alkyl amine?
You can’t. Because the that will form a -2 negative nitrogen which is highly unstable. There is another thing to mention here, your form the negative -1 nitrogen firstly then water or acid is added.
Will the same reactin happen with HCN or will we get CH4 and CH3MgBr like an alkane reaction?
In case of HCN will the same reaction happen or will the H be removed and we will get CH4 and NCMgBr?
why can you not attack a second time with another equiv of grignard? thanks!
The problem is that you’d essentially have a nitrogen with two negative charges on it – very unstable. So it tends to simply do mono-addition.
no i think JARED is asking about why not the ketone formed further react with grignard reagent to give 3 degree alcohol….if it does is it a negative aspect of this reaction?? (if we wanted to get ketone)
The ketone isn’t formed until adding water, which would destroy any Grignard that is present.
I remember that imine salt will generate after nitrile reacts with grignard reagent. Is it possible two equiv of grignard and one equiv of nitrile form the imine salt like R2C-N(MgX)2 ?? Could the negative charges on nitrogen be stabilized by magnesium cation ??
Double addition is rare.
If I do not add acid will the reaction stop at imines
It can especially if the R is aromatic.