Aldol Condensation
Description: The aldol condensation is a reaction between an enolate (or enol) and an aldehyde or ketone that leads to the formation of a new carbon-carbon double bond. This is the product if an aldol addition reaction is heated for prolonged periods. The reaction is called a “condensation” because one molecule of water is formed from the two reactants.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
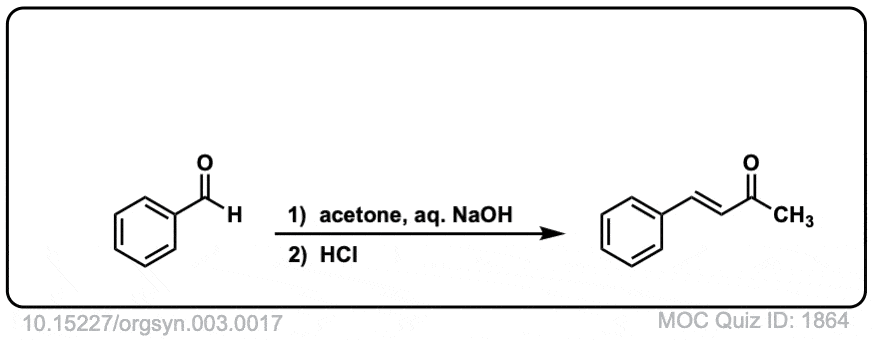
Org. Synth. 1923, 3, 17
DOI Link: 10.15227/orgsyn.003.0017
 Click to Flip
Click to Flip
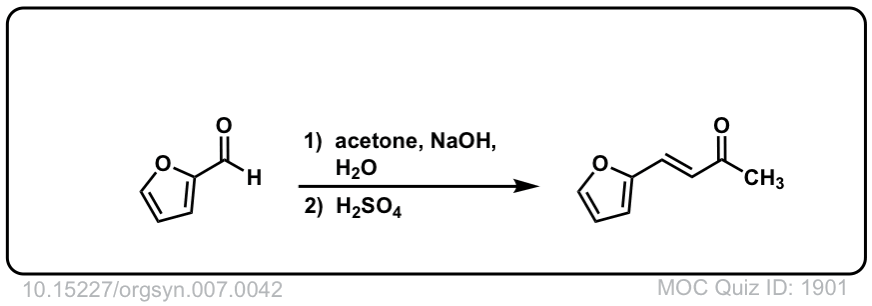
Org. Synth. 1927, 7, 42
DOI Link: 10.15227/orgsyn.007.0042
 Click to Flip
Click to Flip
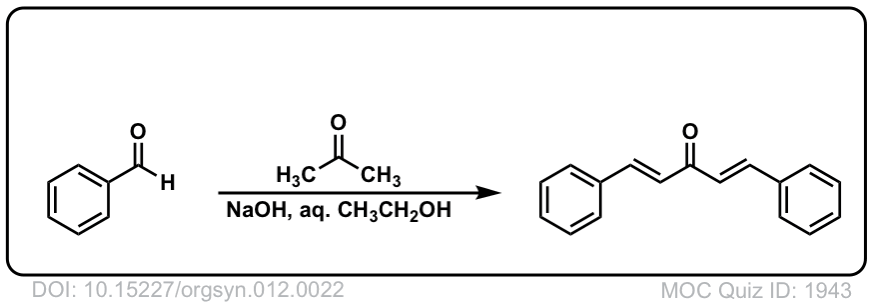
Org. Synth. 1932, 12, 22
DOI Link: 10.15227/orgsyn.012.0022
 Click to Flip
Click to Flip
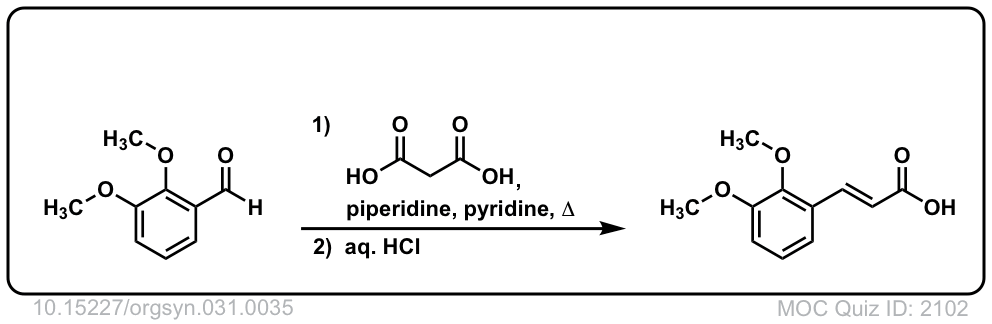
Org. Synth. 1951, 31, 35
DOI Link: 10.15227/orgsyn.031.0035
 Click to Flip
Click to Flip
How do we get the condensation product of the aldol reaction without using heat?
Not sure whether you are reading this from the perspective of a student or of a laboratory chemist.
“Heat” is usually shown for instructional purposes, as a way for instructors to signal when they expect the aldol condensation reaction to be drawn rather than the aldol addition product.
If you give me more context I can give you a better answer.
What are the circumstances in which a condensation rxn would take place without any mention of heat being added? I am super confused as I am running through practice problems in my book and one question, according to the book answer page, leads to condensation. The thing is that there was no mention of heat being added.
Thank you
Without seeing your particular example it’s hard to tell. Only possibility I can think of is if it’s a malonate or other doubly activated enolate (Knovenagel type) in which case elimination is particularly easy since the C-H bond is unusually acidic.
Otherwise I would consider that a problem with the book. Usually students need a clue that the reaction proceeds fully to the condensaation and does not stop at the addition stage. In real life, of course, there is a spectrum of cases, but in a testing environment there should be some way of being able to tell.
in 2nd and 3rd example is it possible that without heat condensation is favour because alkene is in extended conjucation
It’s certainly possible that condensation can happen without heat. From an instructor’s point of view it’s going to be necessary to differentiate between reactions that will lead to addition and those which lead to condensation, and “heat” is the usual way of doing it.
Certainly in the case of benzaldehyde one would expect condensation to be very easy as the result would be conjugaed with the aromatic ring.
Hope this helps! James
thanx James
If water is a solvent, wouldn’t that not be favorable for the elimination of water in the condensation step because of Le Chatelier’s principle?
Good point. You are right that in “real life” water would not be a good choice of solvent for an aldol condensation, both for the reason you mention and also that most organic molecules have poor solubility in water. Many high-boiling solvents could work; the actual solvent is left out, so as to avoid adding confusion.
*hydroxide not hydroxide ion
I thought that hydroxide ion is a very bad leaving group. Why isn’t the hydroxide first protonated by water before leaving?
If done under basic conditions, the hydroxide ion (pKa of water = 14) is actually a weaker base than the enolate ion (pka of ketone = about 20) . So once the enolate is formed, expulsion of HO- is “downhill” since a weaker base is being formed.
Is elimination step in aldol condensation i.e…(step-2) goes through SN1 mechanism?
No. It’s an E1cB mechanism, where the conjugate base (enolate) forms, and then displaces the leaving group.
Why does the deprotonation happen at the carbon adjacent to the carbonyls?
That’s the most acidic C-H bond, because the resulting anion is stabilized by resonance.
Hi James,
the conjugate base of ketone is stabilized by resonance (always) ,
but the alcohol is more acidic in pka table.
how does the deprotonation happen to the ketone here?
thanks in advance
Hi James, I was wondering what would be the favored product in the aldol condensation of 3-ethyl-4-hydroxy-2-hexanone (just my guess how to name the molecule I’m looking at).
Would the product be favored towards 3-ethyl-3-hexenone or 3-ethyl-4-hexenone?
I’m leaning towards the first since the removed hydrogen (assuming a basic reaction) would be from the tertiary carbon.
But… now that I think about it, does base vs acid matter in that case?
Hi Shelby – if I’m reading your question right, the answer is that the double bond would be adjacent to the carbonyl (i.e. on the 3-position). Conjugation increases the stability of the system.
As an additional note, in order for the elimination to occur, you need to form either the enolate (under basic conditions) or the enol (under acidic conditions) and this is going to occur at the 3 position, not the 5 position (as would have to be the case if the alkene formed on the 4-position).
Hope this helps! James
Hi James– can you possibly email me? I have some MS paint drawings that I drew up (I’m very very visual), and I tried sending them in my email reply but I got a message back that says the email cannot be reached.
Being the best site in organic chemistry i was hoping that i could find the mechanism for Cannizzaro reaction (which i wasn’t able to find on googling and referring to Solomon). So can you propose the mechanism for the cannizzaro reaction as well?
Thanks in advance.
Hi – there is a page for the Canizarro, with the mechanism. https://www.masterorganicchemistry.com/reaction-guide/cannizarro-reaction/
In the intramolecular reaction, the ketone you deprotonated has a primary and a secondary alpha carbon, so it has two different enolate forms. The conditions used would encourage formation of the “thermodynamic enolate”, i.e. the more substituent pi bond. Thus, the cyclic product would look quite different. For instance the remaining ketone would not “straddle” the ring but “hang off”, connected to its now tertiary alpha carbon. As I’ve seen it in other sources, a 1,6-diketone should yield a five-membered ring. Is this correct?
While that enolate is in fact the more stable “thermodynamic” enolate, it would form a 4-membered ring, not a 5-membered ring. That’s why it doesn’t happen.
Hi, is the “heat” always important? Or can I use another way for condensation? Thx, P.
Hi Petr – for aldol condensation, heat tends to favour elimination of water. If one wants to perform the aldol condensation, the best way to go about it is to heat the reaction mixture. To avoid the elimination, and to just perform an aldol addition reaction, typically one uses lower temperatures.
Are there any conditions in which the ketone and alcohol remain and the double bond does not form in the final elimination step?
Yes – that’s “aldol addition”. Typically one avoids using heat in that process.