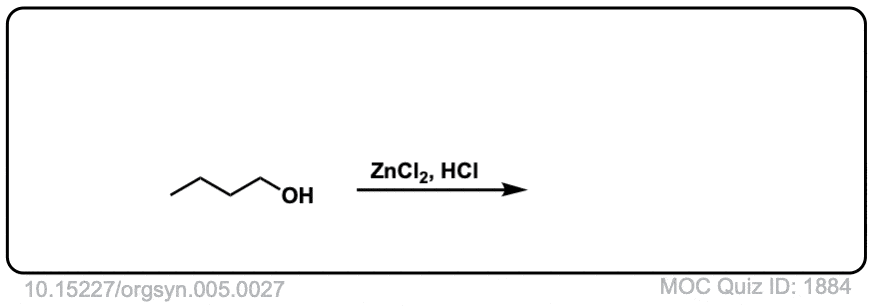
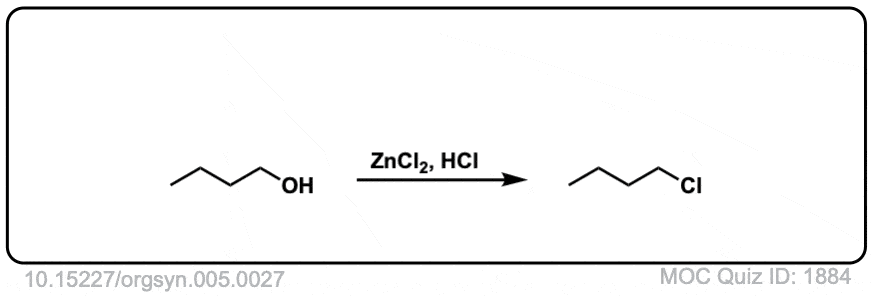
Conversion of alcohols to alkyl halides using HCl
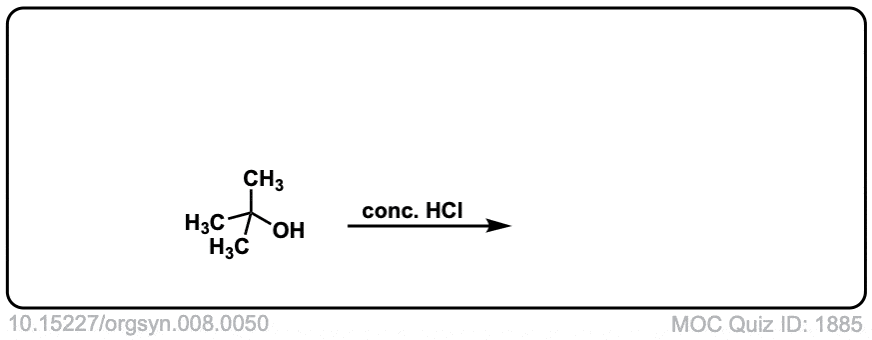
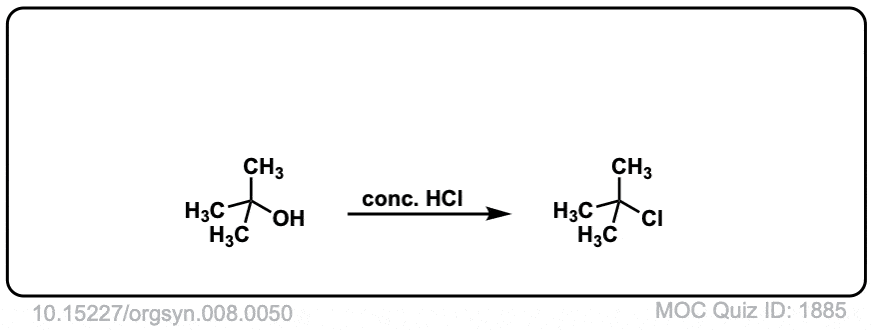
Description: Treatment of tertiary alcohols with HCl leads to formation of tertiary alkyl chlorides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1925, 5, 27
DOI Link: 10.15227/orgsyn.005.0027
 Click to Flip
Click to Flip
Org. Synth. 1928, 8, 50
DOI Link: 10.15227/orgsyn.008.0050
 Click to Flip
Click to Flip
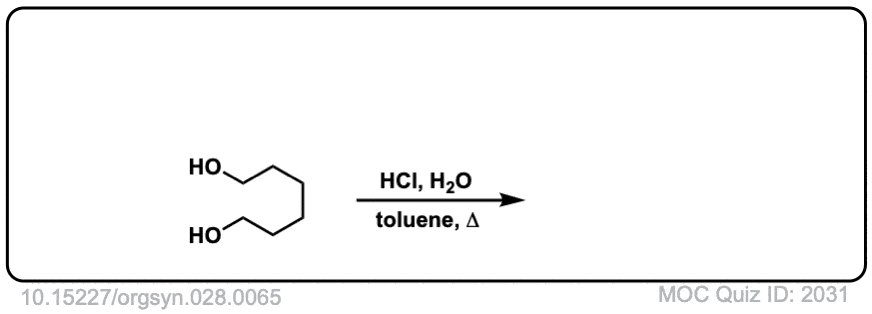
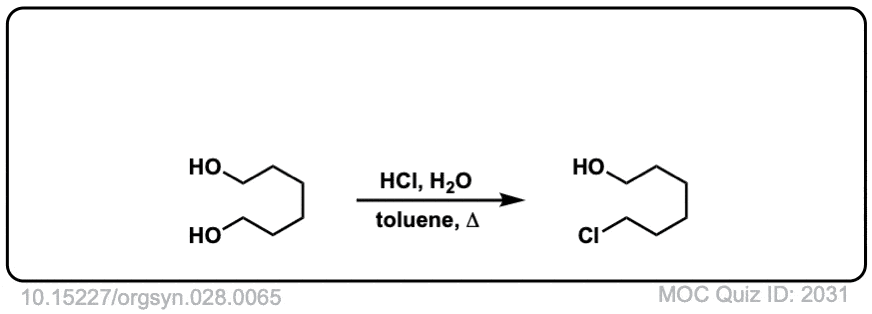
Org. Synth. 1948, 28, 65
DOI Link: 10.15227/orgsyn.028.0065
 Click to Flip
Click to Flip
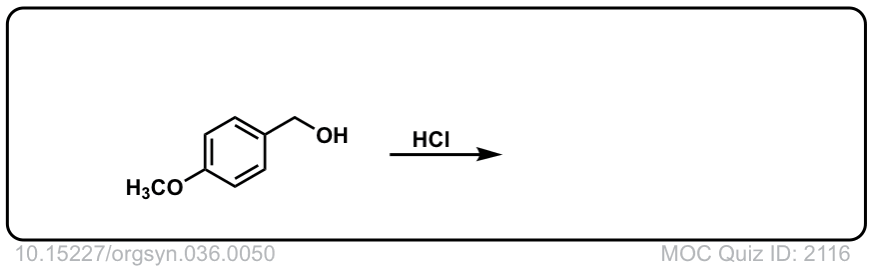
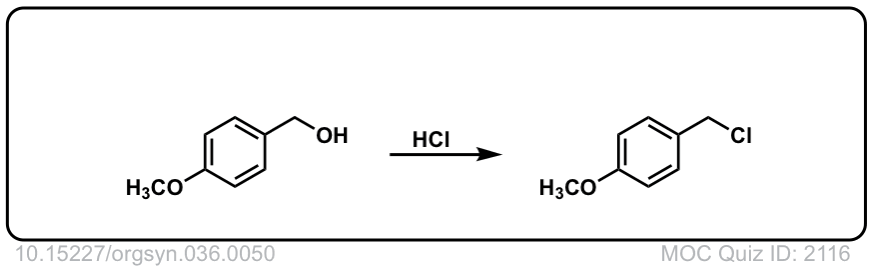
Org. Synth. 1956, 36, 50
DOI Link: 10.15227/orgsyn.036.0050
 Click to Flip
Click to Flip
Although you get a mixture of enantiomers when a chiral carbon is attacked like in example 3 is there a slight preference for the inversion? Or do you get each at roughly 50 50?
In the third example, which product is favored?
How to convert CH3-CHO to CH3-CHBr-CH3?
Methyl Grignard, then HCl.
For the third example, does chirality remain or change?
You get a mixture of retention and inversion, which leads to a mixture of enantiomers. I updated the image to make it more clear. Thanks for asking.
What are the benefits or importance of converting alcohols to alkyl chlorides?
Great question! Alcohols don’t have a good leaving group, so they don’t undergo substitution or elimination (except under acidic conditions). However once you convert them to alkyl chlorides, you can then turn them into all kinds of other useful functional groups.