Elimination (E2) of alkyl halides to give alkenes
Description: Addition of strong base to an alkyl halide results in elimination to form a new C–C π bond.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
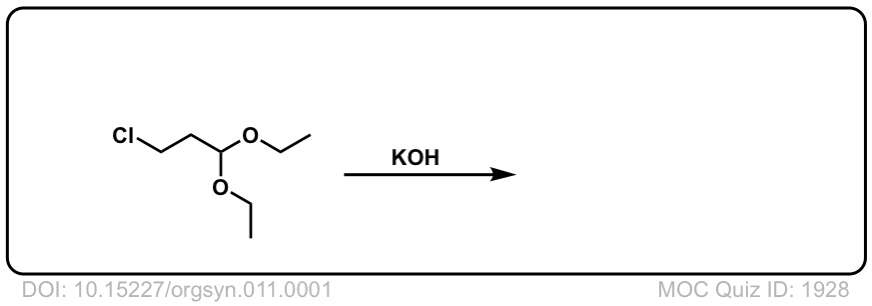
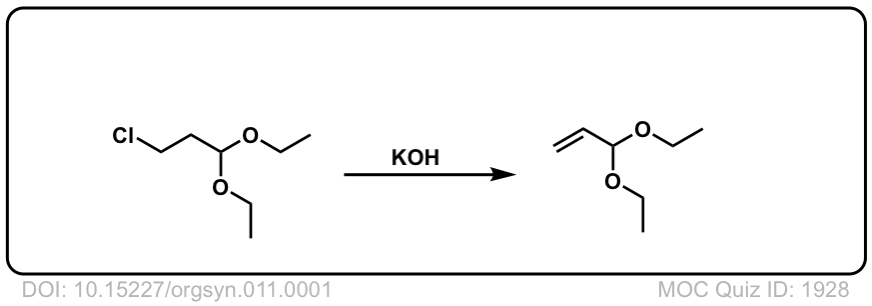
Org. Synth. 1931, 11, 1
DOI Link:10.15227/orgsyn.011.0001
 Click to Flip
Click to Flip
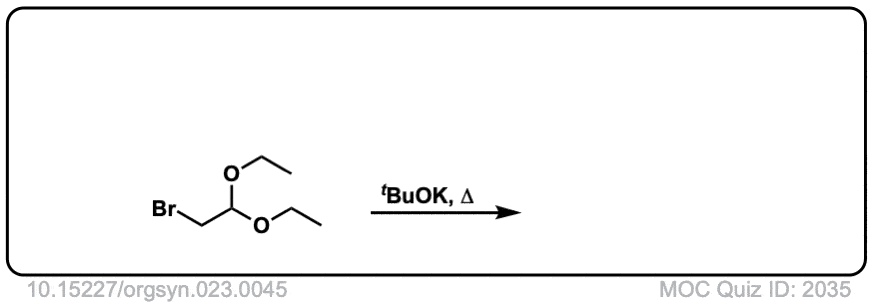
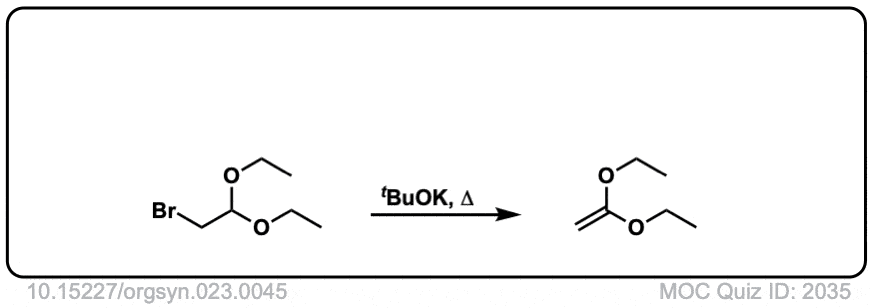
Org. Synth. 1943, 23, 45
Link: 10.15227/orgsyn.023.0045
 Click to Flip
Click to Flip
Org. Synth. 1958, 38, 47
DOI Link: 10.15227/orgsyn.038.0047
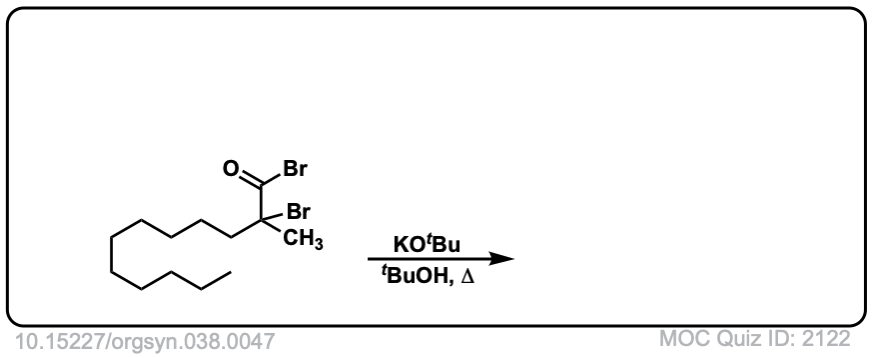 Click to Flip
Click to Flip
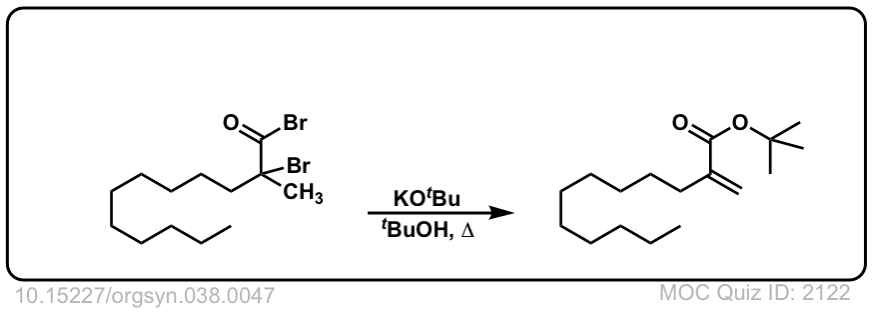
Org. Synth. 1967, 47, 31
DOI Link: 10.15227/orgsyn.047.0031
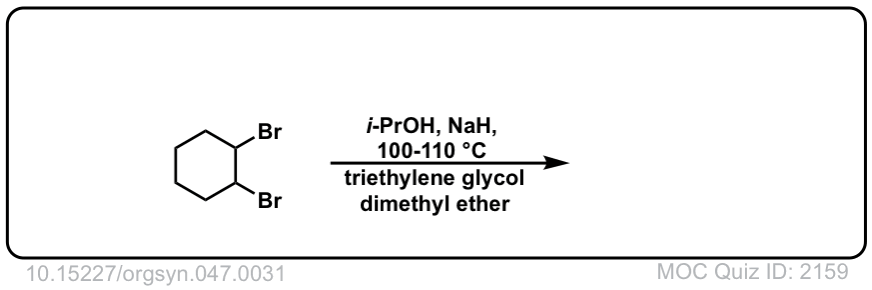 Click to Flip
Click to Flip
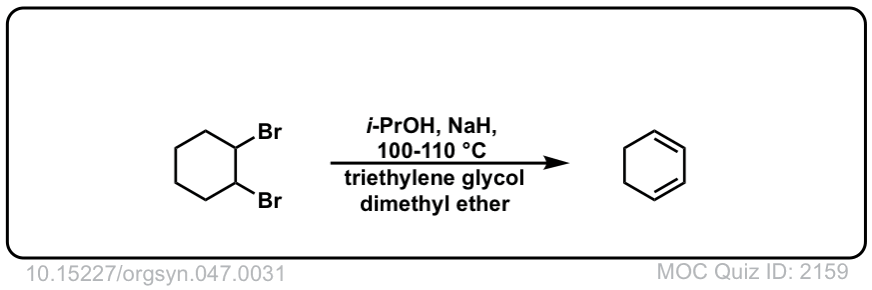
in example 3 is there an implied minor product that is not written?
Yes, I didn’t draw in the less-substituted alkene, the “Hofmann” product. It would be a minor product.
Hihi
on question 6 of the “quiz yourself” Bromine is anti to a methyl group rather than a Hydrogen yet still, the most substituted alken forms. Why is that?
Good question! If you look closely a bond rotation has occurred. The elimination can happen if the C-C bond can rotate, which will then bring the C-H and the C-Br groups anti to each other.
In cyclohexane rings there isn’t free rotation, which is why you will sometimes see formation of the less substituted alkene.
I should probably update the quiz to show the bond rotation.
Hey there, firstly – great website, really helpful! Thanks a lot. Secondly, I was wondering why tertiary alkyl halides give E2 instead of E1 which would generally be preffered due to the carbocation stability?
With strong base, E2 will be favored over E1. Rate determining step in E1 requires formation of carbocation which has a high activation barrier. E2 doesn’t require formation of a carbocation and is generally quite fast.
Hello, can you elaborate more on example 2. In the first example we’ve seen that the carbon pi-bond is formed where it is more stable. If we follow the same rule, the pi bond that is formed in the example 2 should be between C1 and C2. You said that’s because of the that the leaving group and the hydrogen have to be anti to each other in order for elimination to occur, and the methyl group is on C1 which stops it. However we do still have an available H-atom on C1 which can help the bonding to occur between C1 and C2. I’m so sorry but maybe it was meant to be a double bond between C1 and C6?
Hi! That example is correct. It’s an E2 elimination. Ideally the most substituted alkene would form, but this isn’t possible since the Cl is anti to a methyl group instead of an H on the more substituted carbon.
Therefore the elimination must happen on the other beta carbon which actually does have an available H that is anti to the Cl.
The H- atom attached to the carbon bearing Cl can’t participate in the E2 elimination. That would be what is called an “alpha-elimination” and although they are known (e.g. from HCCl3 in the presence of strong base) the carbon isn’t acidic enough for that to happen here.
What about primary alkyl halides? How do you turn them into Alkenes?
Usually treatment with a bulky base will do the job. The classic example is t-BuO(-). In some cases tBuO(-) will also do the SN2 reaction (especially with unhindered primary alkyl halides like CH3CH2Br or CH3CH2CH2Br). Another option is a weak amine base like pyridine, triethylamine, or even (if your course covers it) DBU.
Question: if isobutyl bromide is treated with -OH and H2O would this result in an E2 and SN2 reaction? I’m assuming that even though H2O is a polar protic solvent and -OH is a strong nucleophile the H2O would not affect -OH since it is -OH’s conj. acid. I’m thinking in this case with no heat added you would observe a competition between the -OH anion either deprotonating the single H from the beta carbon acting as a Lewis Base or a substitution resulting in an attack at the alpha carbon and replacement of Br with -OH? I guess seeing both -OH and H2O threw me off.
Very helpful thank you!
the reaction is 100 percent anti and the halide ion and the hudrogen atom that is polarised by the strong base shouid be diametrically opposite and the molecules periplaner . it seems to be a reaction of two steps bt is single step reaction