Oxidation of Primary Alcohols to Aldehydes using PCC
Description: Treatment of alcohols with PCC leads to formation of the aldehyde.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Time Example:
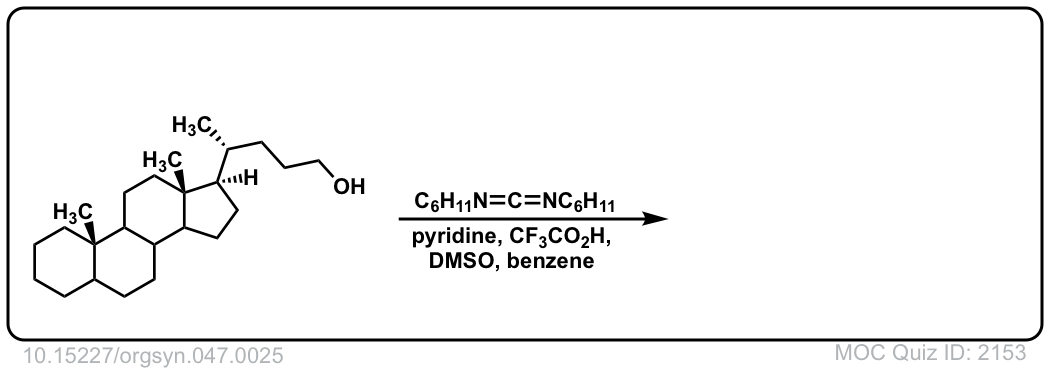
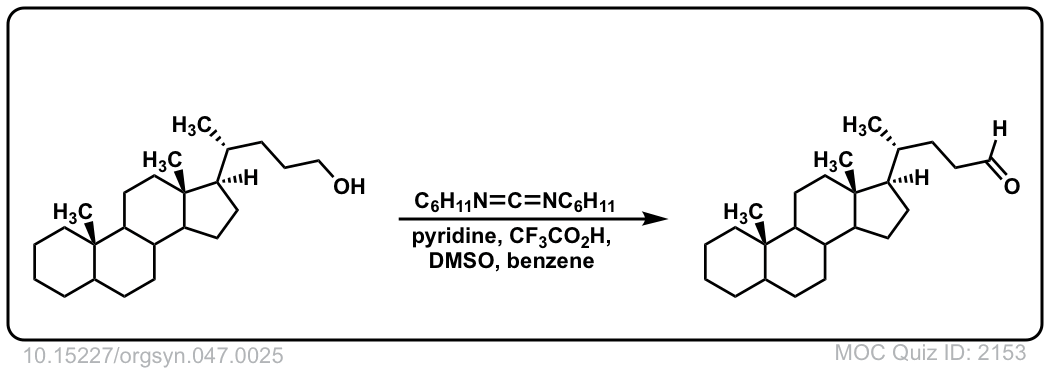
Org. Synth. 1967, 47, 25
DOI Link: 10.15227/orgsyn.047.0025
 Click to Flip
Click to Flip
I used PCC to oxidize the alcohol but I didn’t get result and I don’t know what should I do? Can any one tell me wheres the problem?
It’s hard to answer a question like this without more specific details. There are many possible reasons why the reaction might not work, starting with your substrate, but also potentially depending on solvent, reagent quality, presence of water, etc.
Hi…i m trying to oxidise primary alcohol by PCC bt the compound is insoluble in DCM what can i do…
The original procedure calls for DMF.
As it is pictured the proton transfert in step 2 involve an intermediate that is highly unstable (4 member ring). Does it really happen that way?
Good eye. No, it likely does not, although it could. I took some liberties with the proton transfer steps because if they were all drawn properly the mechanism would be so long that, in my opinion, many students would not find it useful.
oxidation of CH3CH2CH2C(CH3)2CH2OH
Convert that alcohol on the end to an aldehyde.
and what might be a solvent then
Solvent for this reaction is usually CH2Cl2 or DMF.
check the last step of the mechanism, you accidentally made the hexane ring into a benzene ring
Hi there,
Why is it that oxidation of ALDEHYDE will not happen under PCC? What is exactly the reason for that? We have a hydrogen in aldehyde, how come that does not get oxidized?
and why is that you say “as long as” Water is excluded? does that imply we can deliberately introduce water do the system to further oxidize the aldehyde if we want to?
Good question!
Water is key.
If water is not added, the aldehyde will not oxidize.
However, if water is added, it will add to the aldehyde to form a hydrate (carbon with two OH groups attached). And this will undergo oxidation, (through a mechanism similar to that of a primary alcohol) to give a carboxylic acid.
if we add water then how we stop reaction at aldehyde stage while converting primary alcohol to aldehyde
Generally you don’t want to add water in this reaction, except in the workup stage when trying to isolate the product.
how to decompose the tar obtained after using the PCC as an oxidising agent in the reaction
Best approach is to prevent tar formation by adding molecular sieves or Celite to the reaction; the tar will stick to that instead of the flask.
Thanks for the tip. Would you happen to know the best way to get the chromium out of the sample afterwards? I was going to filter it through Celite, but if that’s already in the reaction vessel, would it even be necessary?
Generally I’d suggest filtering through a pad of fresh Celite to get rid of the chromate ester byproducts and other crap; typically rinse with CH2Cl2. You’ll find that the brown color will be very difficult to remove, however, without purification through a plug of silica or by distillation.
Also, recently found this tip:
” Quench with 1M citric acid: it complexes all the brown Chromium junk. Much better than the usual “Celite filtration” work up.
God bless E J Corey :-)
Nice tip james, but normally DMC is used as the solvent for PCC oxidation, how did you use mix 1M citric acid water solution with it to complex the brown junk and how to get rid of residual citric acid? Looking forward to your answer. Thank you.
DMC? Do you mean DMF?
DCM=Dichloromethane
PCC reaction is always tough in terms of the formation of sticky chromium junk. Many of times product loss is also happened.
The best solution I found is similar to that of E J Corey. If you use 1g of PCC, use 1.3g of silica gel (230×400 mesh) or (60×120 mesh). Add your alcohol solution in DCM dropwise.
This would surely avoid the formation of sticky material and improving yield. After completion of reaction if you check RB flask, you will find that there is no sticky junk. If you are lucky and if your product is soluble in diethylether then add dropwise 1.5 volume of it with respect to dichloromethane. It will release all chromium junk soluble in dichloromethane. Easily filter through celite pad or sintered funnel. Wash with diethyl ether. This will do your maximum work.
Alternatively, if your compound is liquid/solid and also soluble in hexane. Add dropwise hexane (2-3 volumes with respect to dichloromethane volume), stir and filter through celite or sintered funnel. Believe me, you won’t need further purification of aldehyde and there won’t be any over oxidation and any other side reactions as PCC has no reactivity in hexane.