Oxymercuration of Alkynes
Description: Alkynes treated with mercury (usually HgSO4) and water will be hydrated to give ketones, via an enol intermediate.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
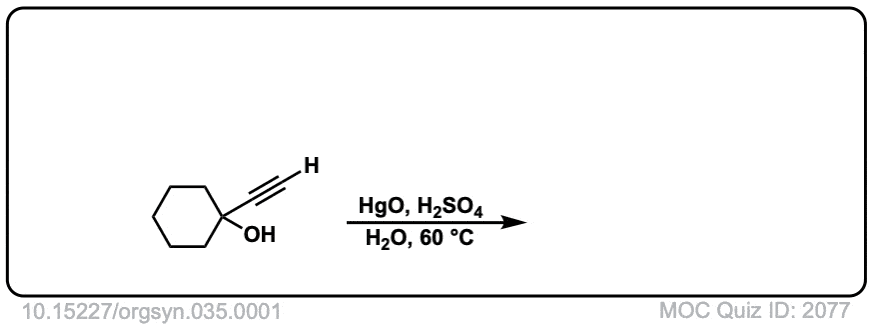
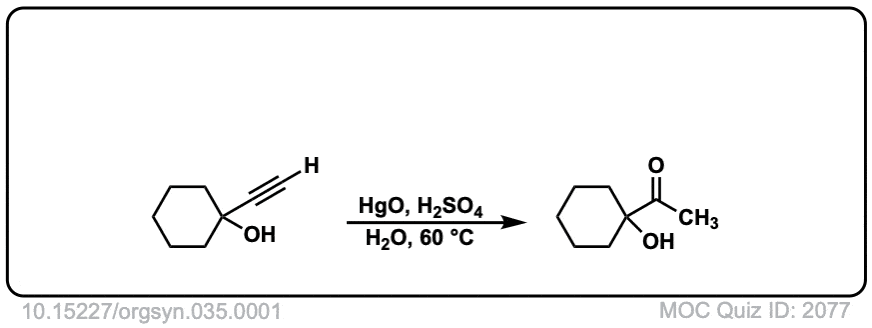
Real-Life Example:
Org. Synth. 1955, 35, 1
DOI Link: 10.15227/orgsyn.035.0001
 Click to Flip
Click to Flip
During Oxymercuration demercuration of ethyne, is it true that tautomerism does not occur and product is only in enol form.
Tautomerism should occur! The main product would be acetaldehyde.
Without the use of HgSO4, the rxn shows very poor yeild. But with HgSO4 we get high product yeild. What is the reason for this.
With H+ there is no alternative but to form a vinyl carbocation, which is very unstable. With a large Lewis acid like Hg2+ a bridging carbocation can form, avoiding a carbocation on an sp2 hybridized carbon. Much more stable.
we can use HgSO4 with dil.H2SO4
Yes, we can.
we can use Hg(NO3)2
Yes, that would also work.
if we use Br2 and H2O to react with terminal Alkyne,it will yield ketone. How it works?
Forms an enol, and then tautomerization to give the ketone.
Can we use Hg(NO3)2 in oxymercuration?
Not sure I’ve seen that reagent in an oxymercuration, but I don’t see why not. Should work well.
What is the major product formed on reaction of pent-2-yne with H2SO4 and HgSO4?
You’ll get a mixture of 2-pentanone and 3-pentanone.
Will HgSO4 have an effect on a double carbon bond?
Yes – it will work just as well as Hg(OAc)2 . For alkynes we generally use HgSO4 because the reaction is done in concert with H2SO4, which helps to remove the mercury from the substrate.
Given that the cyclic mercuronium ion formed in step 2 has lone pairs of electrons on it and that resonance is possible, shouldnt the ion be anti aromatic and unstable? Is it possible that the intermediate in step 2 is a vinylic carbocation?
The C-Hg bonds are very long and orbital overlap between any empty p orbital on Hg with the carbon p orbitals is likely very poor. Antiaromaticity isn’t really a concern here.
If I remember organic I correctly HgSO4 is only required for terminal alkynes so that the reaction is carried out under more mild acidic conditions, and that for internal alkynes it isn’t necessary but often is carried out in this manner as well (the HgSO4 component, that is)?
With HgSO4 the reaction is milder than with straight acid (e.g. H3O+) although in truth more advanced methods these days avoid mercury and use late metal cations such as Au and Ag