Reactions of Diazonium Salts
Description: Treatment of diazonium salts with various reagents leads to formation of a new bond to the aromatic ring and loss of nitrogen (N2).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
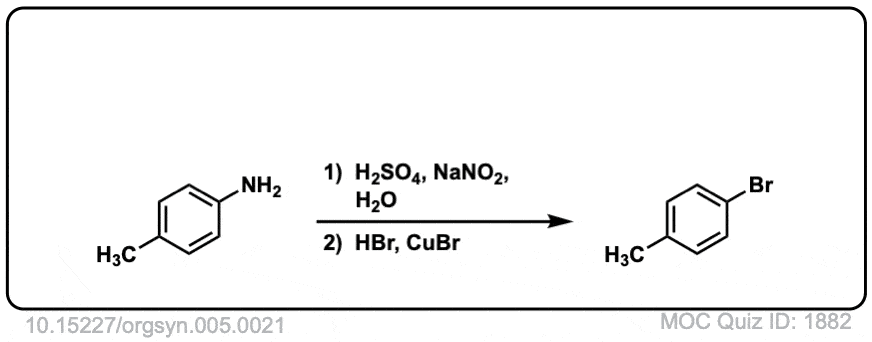
Org. Synth. 1925, 5, 21
DOI Link: 10.15227/orgsyn.005.0021
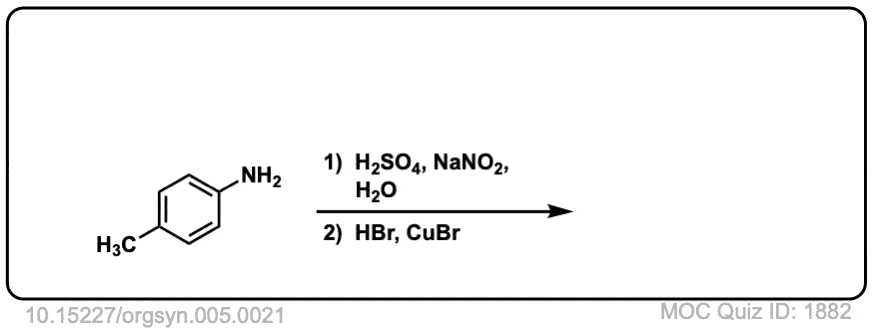 Click to Flip
Click to Flip
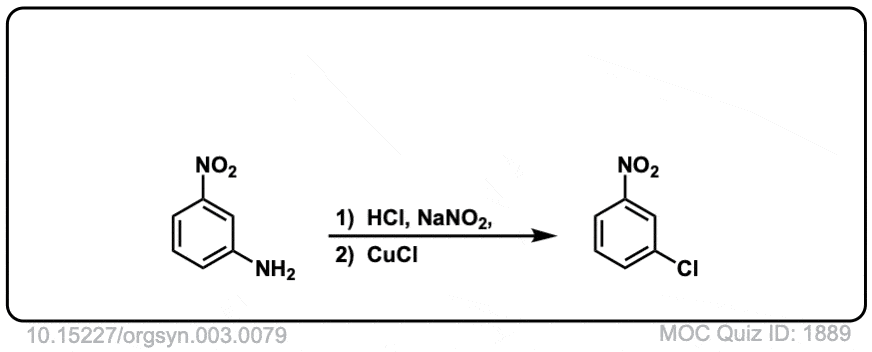
Org. Synth. 1923, 3, 79
DOI Link: 10.15227/orgsyn.003.0079
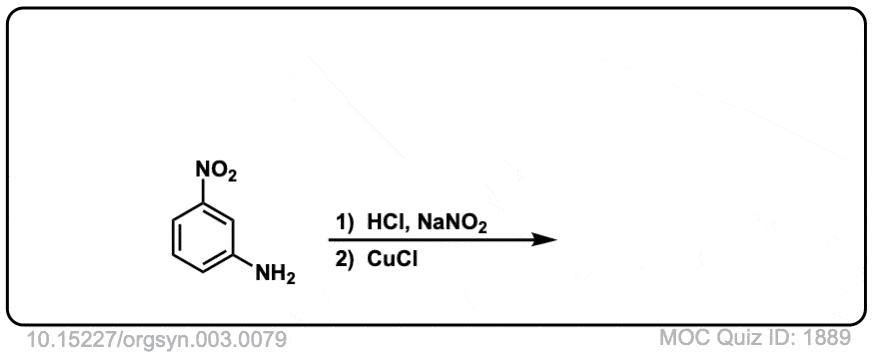 Click to Flip
Click to Flip
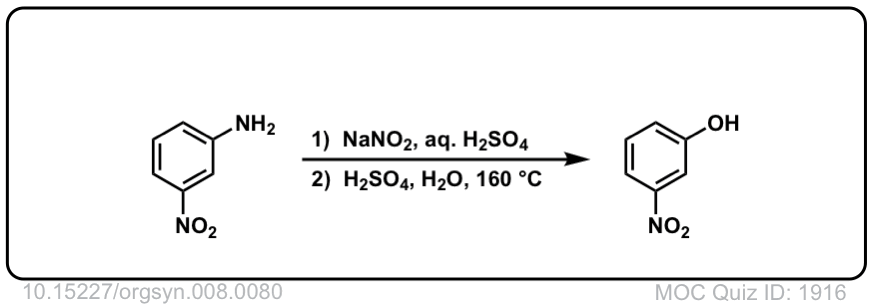
Org. Synth. 1928, 8, 80
DOI Link: 10.15227/orgsyn.008.0080
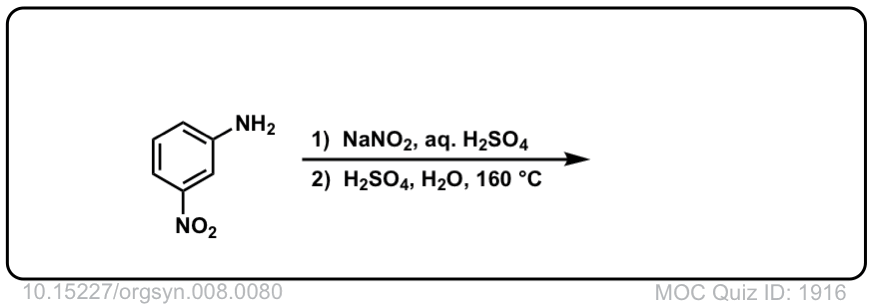 Click to Flip
Click to Flip
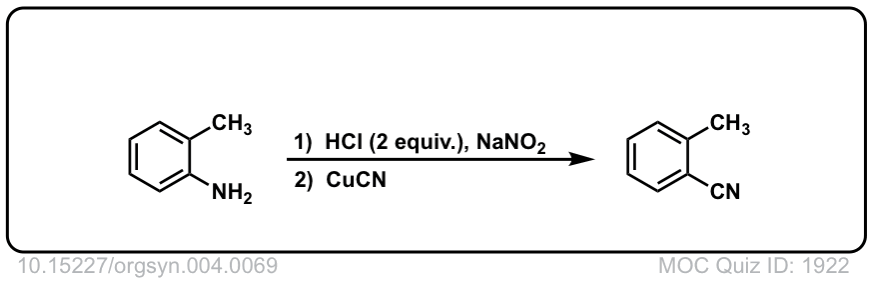
Org. Synth. 1925, 4, 69
DOI Link: 10.15227/orgsyn.004.0069
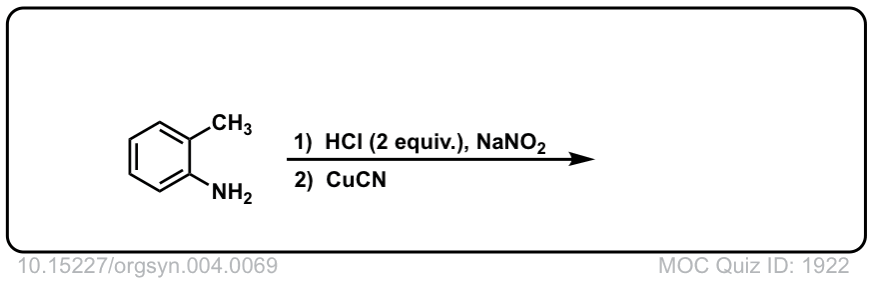 Click to Flip
Click to Flip
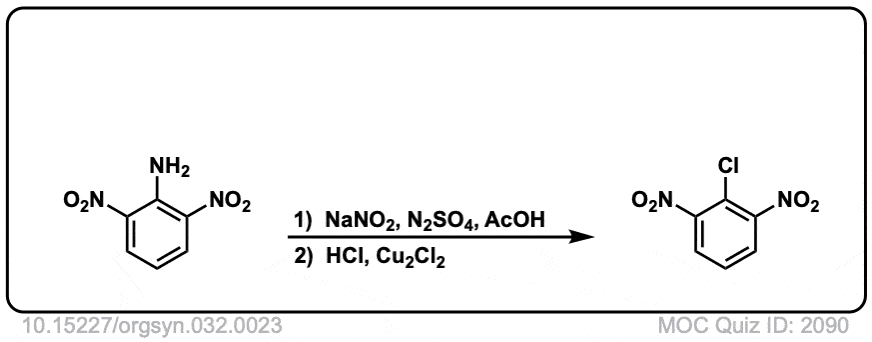
Org. Synth. 1952, 32, 23
DOI Link: 10.15227/orgsyn.032.0023
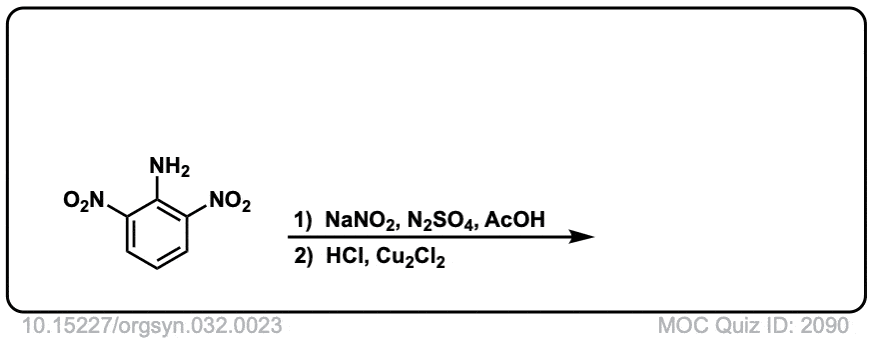 Click to Flip
Click to Flip
Hi, is the carbon C, where is bonded -N+N, the same carbon where is bonded the X from the Description? Thx, P.
Yes, it should be!
Hi, in the Examples the example 4: Ph-N+N + H3PO2 —> “benzene”. Why in the reaction Ph-N+N + HPO —> is produced “phenol”? Its is ok the other reaction? Thx, P.
You mean H3O+ ?
No, I mean acid HPO.
In the reduction to benzene, should the reactant be H3PO2 instead of H2PO3? That is how I have seen it elsewhere.
Shoot. Fixed. Thanks!