Reduction of nitriles to primary amines with LiAlH4
Description: Lithium aluminum hydride [but NOT sodium borohydride (NaBH4)] can reduce nitriles to primary amines.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples
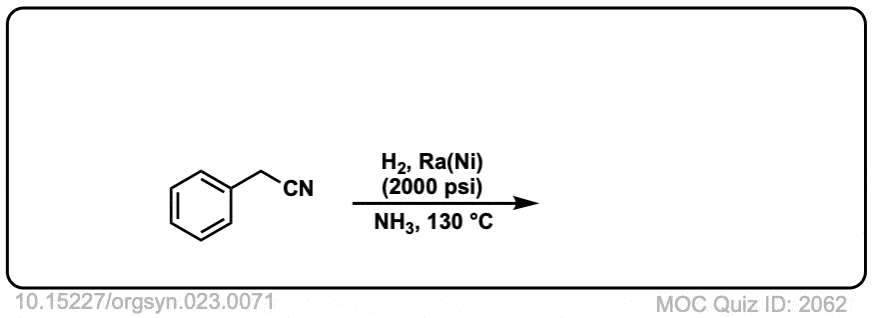
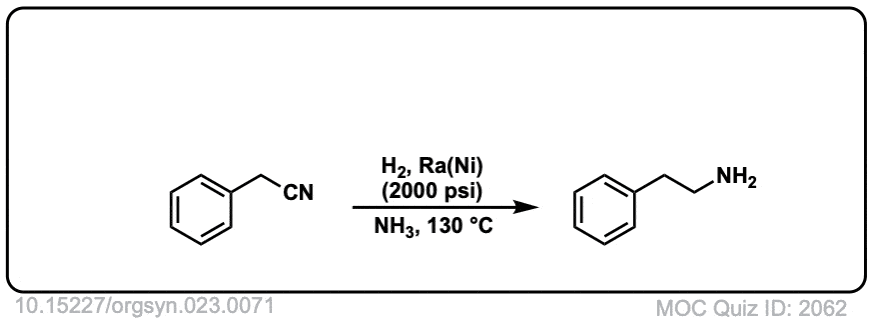
Org. Synth. 1943, 23, 71
DOI Link: 10.15227/orgsyn.023.0071
 Click to Flip
Click to Flip
Can Nitriles be reduced to amines using Sn/HCl
I don’t believe so. None of my references say it will. It will reduce nitriles to imines however.
One of the nicest and cleanest ways to reduce nitriles to amines is to use NaBH4 in the presence of a cobalt catalyst, CoCl2.
Can H2,Pd/C also reduce the cyanohydrin?
Not the best, but it *can* be done with H2 and Pd/C, if the temperature is high enough. From this (German) paper: “The previously described hydrogenations of nitriles with bound hydrogen and Pd / carbon succeed at sufficiently high temperature even with elemental hydrogen. α-Cyan-cinnamic acid esters are converted into α-methyl-hydrocinnamic acid esters.”
https://onlinelibrary.wiley.com/doi/abs/10.1002/jlac.19677070107
Very misleading. Sodium borohydride is regularly employed to reduce nitriles in combination with transition metals (e.g. Ni, Co).
But that’s not the same as NaBH4 – that’s in situ formation of nickel or cobalt hydride! Completely different. Also – keep in mind that this is written for introductory organic students who encounter NaBH4 and LiAlH4 but will never encounter NaBH4 in the presence of nickel or cobalt salts.
For step 2. Instead of N- getting a second negative charge N (2-), would it first protonate itself once? and then repeat from there until it left?
Hey Matt – although N is drawn with a second negative charge in practice it will be bound to aluminum [an excellent Lewis acid], and the Al-N bond will be quite strong so it’s not as “naked” as it appears. There aren’t really any proton sources present to protonate the nitrogen at this stage until workup.
Yeah, that makes sense, basically the electron density of N is being donated to Al, resulting in less anionic nature as observed.
Thanks James!
GOOD WEBSITE FOR LEARNING