SN2 reaction between azide ion and alkyl halides to give alkyl azides
Description: Alkyl halides (or tosylates) will react with azide ions (such as NaN3 or KN3) in an SN2 reaction to give alkyl azides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
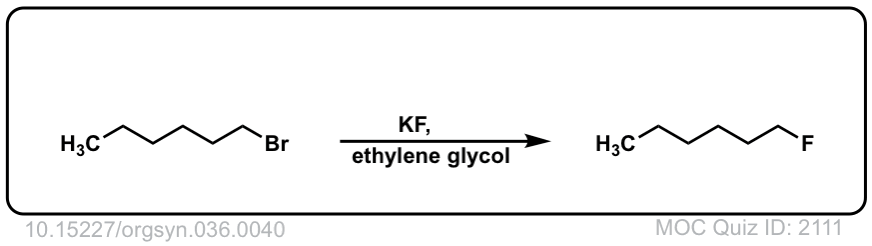
Org. Synth. 1956, 36, 40
DOI Link: 10.15227/orgsyn.036.0040
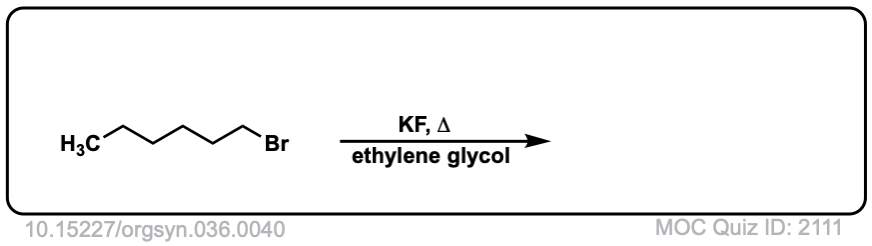 Click to Flip
Click to Flip
Org. Synth. 1965, 45, 47
DOI Link: 10.15227/orgsyn.045.0047
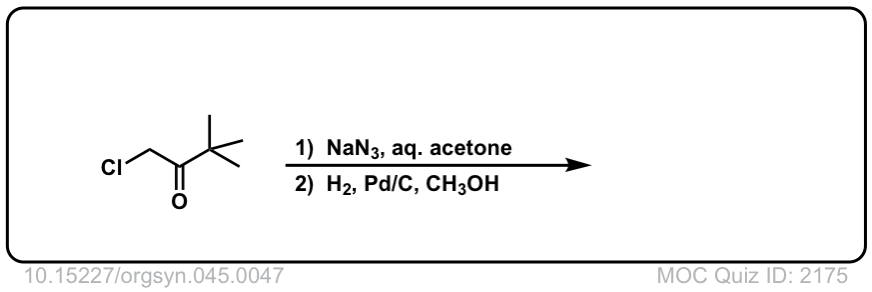 Click to Flip
Click to Flip
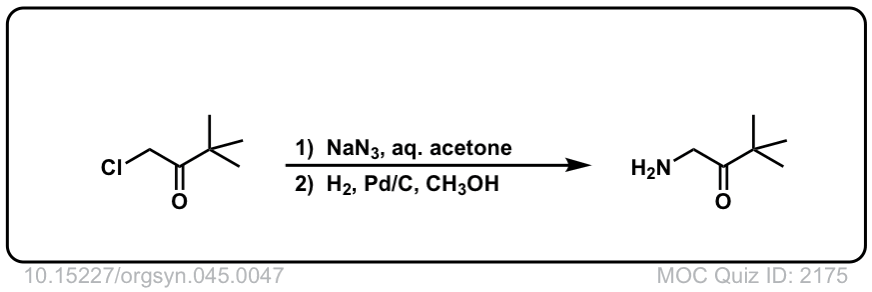
Hello, for the last quiz, DOI Link: 10.15227/orgsyn.045.0047, the H2/Pd /C should reduce the ketone group to an alkane, right?
Not necessarily. It is very possible to selectively reduce azides to amines with Pd-C / H2 without reducing ketones.
Hi, thanks for the guide. very useful!
Mistake on Example #2: salt formed should be KCl, not NaCl.
Is an alkyl halide formation possible from an alkyl azide?
I’m not sure. I’d be a little squeamish about using strong acid or Lewis acids because the intermediate HN3 or metal azides can be pretty unpredictable.
Hello! Wonderful summary sheets, I really appreciate the straightforward explanations. I think there’s a typo on this page, the last example shows a bromide where the azide should attach, right?
Fixed, thank you!
Fixed. Thanks!