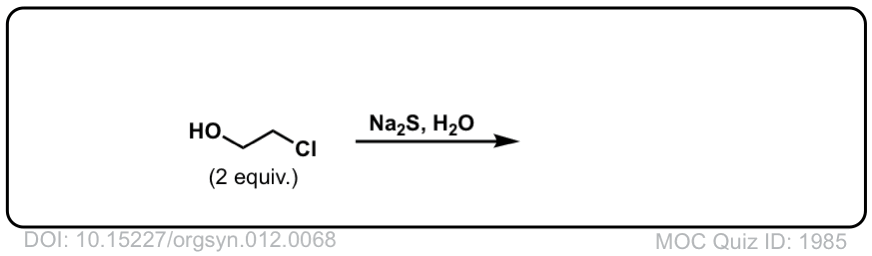
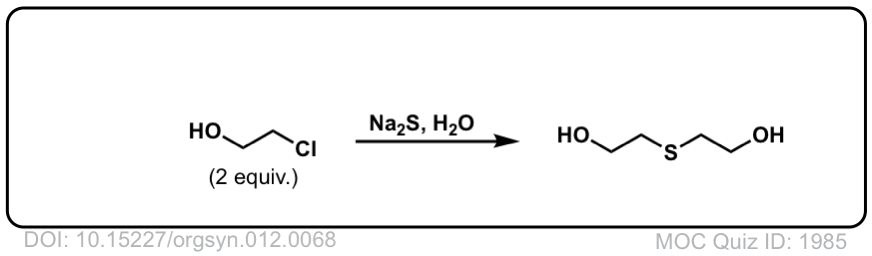
SN2 reaction of hydrosulfide ion with alkyl halides to give thiols
Description: Alkyl halides (or tosylates) will react with the hydrosulfide ion (HS–) to give thiols.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1932, 12, 68
DOI Link: 10.15227/orgsyn.012.0068
 Click to Flip
Click to Flip
Hi, I was wondering how I could access the mechanism for the quizzes you post for each reaction.
Don’t you have to use DMSO as the solvent for the second example?
A polar aprotic solvent like DMSO or acetonitrile would certainly be helpful for these reactions. It’s unlikely to have any competition with the E2 since the sulfide anion is not particularly basic (good threshold is the E2 doesn’t compete if the pKa of the conjugate acid is 12 or below. Thiols have a pKa of 12).
The explanations given here are point perfect and there is no need for other references.