Sulfonation of Arenes to give sulfonic acids
Description: Treatment of an aromatic molecule such as benzene with sulfur trioxide and a strong acid leads to formation of sulfonic acids.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
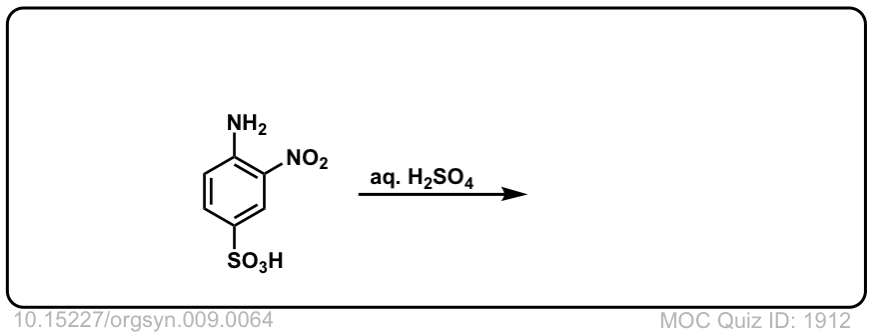
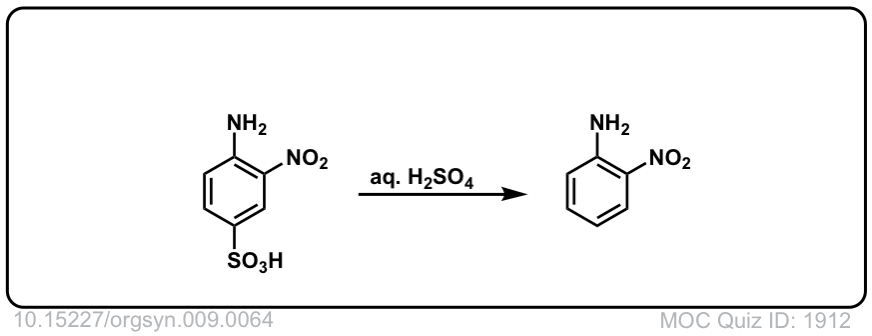
Org. Synth. 1929, 9, 64
DOI Link: 10.15227/orgsyn.009.0064
 Click to Flip
Click to Flip
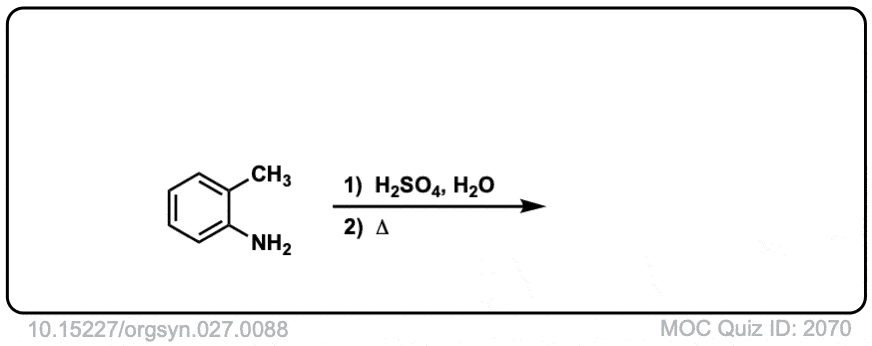
Org. Synth. 1947, 27, 88
DOI Link: 10.15227/orgsyn.027.0088
 Click to Flip
Click to Flip
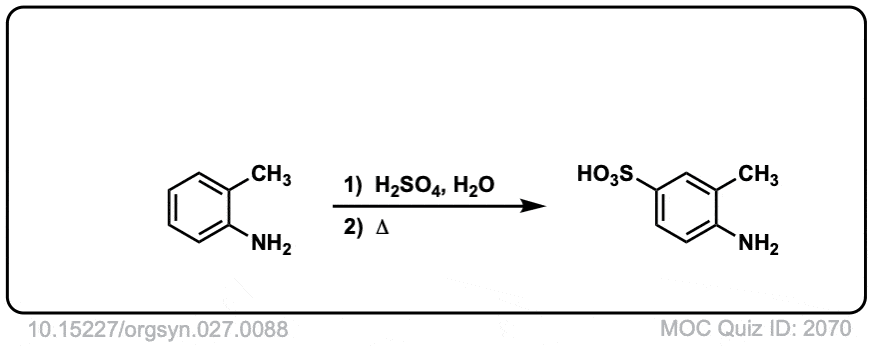
How does Org. Synth. 1947, 27, 88 work? There isn’t any SO3 to make the sulfonic acid.
Great question! Looks like they make the sulfate salt of the aromatic amine, and then heat it to 180°C under reduced pressure. Under these conditions it must rearrange to give the arylsulfonic acid.
They say the prep is based on Helv. Chim. Acta 1932 – https://onlinelibrary.wiley.com/doi/10.1002/hlca.193201501163
Possible mechanisms are given in this paper (PDF is open access) – https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.54.3048
This is definitely beyond typical “intro to organic chemistry” material.
Since HSO4- is the conjugate base of a strong acid, how does it strong enough of a base to eliminate the proton (Step 3) to reform the aromatic benzene? I have seen a similar but different mechanism for this reaction that avoids this problem by initially having the pi bond attack SO3 rather than SO3H, resulting in an anion, at which point water acts as a base deprotonating to reform the aromatic system. This water is now a hydronium ion which is used to protonate the SO3- to become SO3H. How do I know which of these two mechanisms is correct?
The proton in question is extremely acidic as its conjugate base is quite stable (it’s aromatic).
If neutral water is present, then yes, neutral water would likely act as the base here.
Protonation of SO3 to give SO3H+ accelerates the reaction considerably. The rate limiting step is not deprotonation, but attack of the benzene ring on sulfur.
Why does benzene pie attack the S? It already has 6 bonds?
It’s not easy to do – but it happens. It’s much more difficult to perform this reaction on benzene than on an ordinary alkene. It’s usually important to heat this reaction.
Wonderful. Thank you. Electron withdrawing due to the electronegative oxygens, and the fact that the Pi orbitals are not the same size? Would it be useful as an instigator for addition-elimination nucleophilic aromatic substitution? (the same way that NO2 is?)
Does sulfonation activate the ring for future electrophilic aromatic substitutions?
No, SO3H is an electron withdrawing group so it actually deactivates the ring. Hope this helps! James
i want to prepare sulfonic acid at small scale industry.pl give us details.
You should hire a consultant.