Wolff Kishner Reaction – conversion of ketones/aldehydes to alkanes
Description: The Wolff-Kishner is a reaction for converting carbonyls (such as ketones and aldehydes) into alkanes.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
Org. Synth. 1958, 38, 34
DOI Link: 10.15227/orgsyn.038.0034
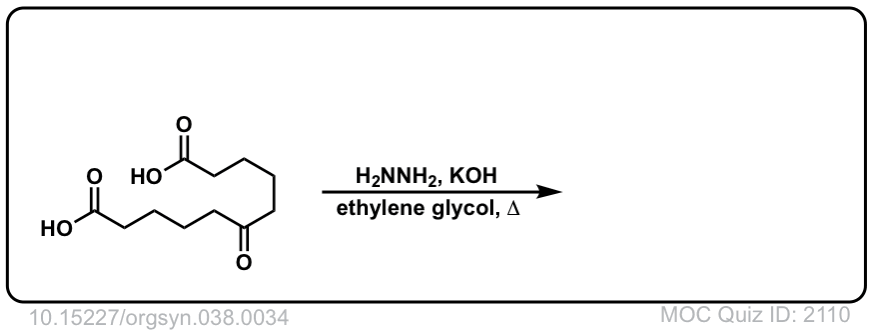 Click to Flip
Click to Flip

Org. Synth. 1967, 47, 83
DOI Link: 10.15227/orgsyn.047.0083
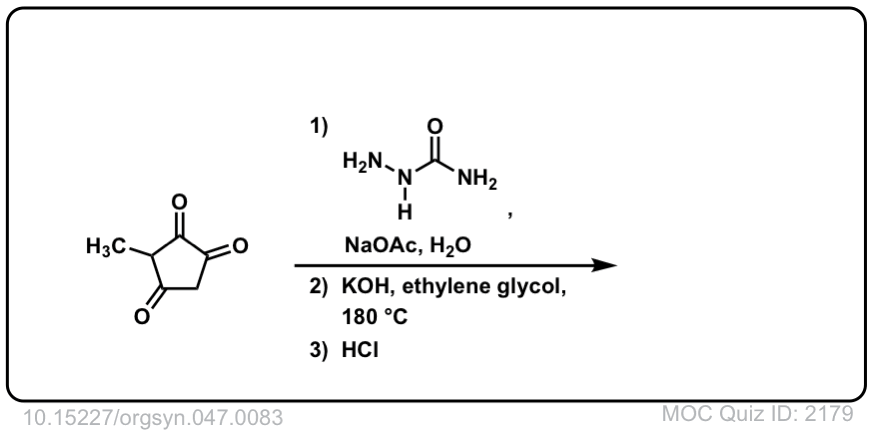 Click to Flip
Click to Flip
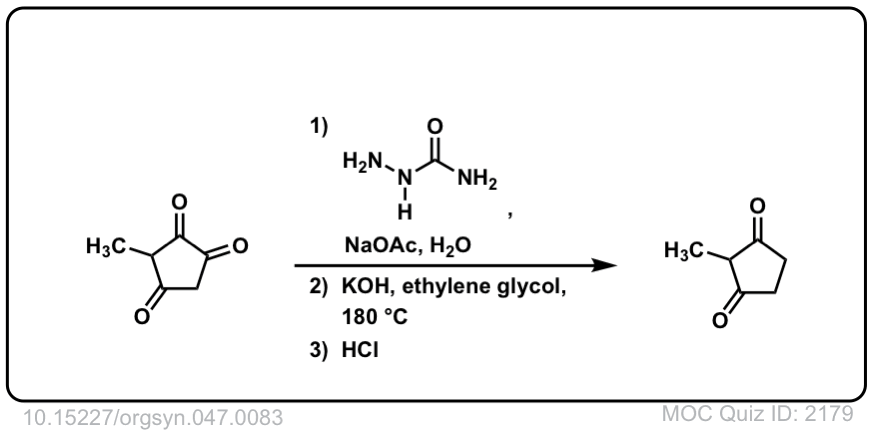
Why don’t I use heat in the last two examples?
Heat should probably be in there.
Sir, what will be the product if we imply wolf kishner reaction on carboxylic acids with ketone group like
CH3-CO-COOH.
Does it will just reduce ketone group only or will react will acid group also?
Please reply!
Not going to work well with a free carboxylic acid there.
My professor won’t allow us to transfer Protons intramolecularly….. Do you know why is that…..?
Probably because the acid and base need to be able to reach each other, which is generally not possible unless they are 5 or 6 atoms apart. So I would use an external base / external acid to perform the proton transfer.
In fairness that is probably a better way to do it, but it takes forever to write out. My approach is a bit of a short cut.
Yes – angle strain. See Proton Transfer
Isn’t there a acid catalyst here in this reaction. So we will have the OH protonation (so now the leaving group it’s H3O+ before 1-2 addition of N. please kindly correct me if you think I am wrong.
There could be an acid catalyst for the formation of the hydrazone, but once the hydrazone is formed the Wolff Kishner itself is performed under basic conditions.
Hi James,
The reaction will be the same if I were to use methanol instead of water, right? Thank you in advance!
If you mean the base will be the conjugate base of ethylene glycol instead of HO(-), then yes, that is correct!
what happens if there’s a Nitro group in the compound? does it convert to nh2 ??
No, it should not. Although a nitro group would be reduced by Pd-C/H2 and also the Clemmensen reduction.
oh- a bad l.g can leave because it’s a catalyst?
Isn’t there a acid catalyst here in this reaction. So we will have the OH protonation (so now the leaving group it’s H3O+ before 1-2 addition of N. please kindly correct me if you think I am wrong.
Would this mechanism be the same for aromatics?
Yes, it would work exactly the same way for aromatic and non-aromatic ketones.
Wolf kishner reaction possible in carboxylic acids or not?
No, strong base deprotonates the carboxylic acid, it’s too difficult to form a hydrazone on that species.
Will this reduce a NO2 substituent on the same ring?
No, the Wolff-Kishner will not reduce a nitro group. You’d want to use a metal such as Fe or Sn in the presence of acid, or catalytic hydrogenation.
Does Wolff Kishner reduce alkenes?
No it does not. Although if you start with hydrazine, you can oxidize it in situ (e.g. with H2O2) to “diimide”, which is a metal-free method for the reduction of double bonds. See here: https://en.wikipedia.org/wiki/Reductions_with_diimide
In the Wolff kishner we first convert ketone or aldehyde to hydrazone. Hydrazone formation is done in acid medium. Now if we have a carbonyl compound with an Acetal and Ketone, will the acetal be also reduced in Wolff kishner due to equilibrium being established in during hydrazone formation (acid medium form ketone and ketone formed along with original one will react to form two Hydrazone sites, which can be reduced in proceeding step with strong base. Couldn’t find any answer to this.
Thanks in advance!
Acetal hydrolysis requires aqueous acid. Hydrazone formation is done using anhydrous acid. So the acetal should be unaffected by these conditions.
It really makes sense, low H2O – complete acetal side of equilibrium. Thanks from saving me from a headache. And great site.
nice presentation. Can we use potassium carbonate and ethylene glycol and temperature ~200 degree C?
I suppose you could use potassium carbonate but it will not deprotonate ethylene glycol irreversibly; at 200 degrees C, however, the reaction should still proceed well.
And what is a role of ethylene glycol in this reaction?
The site is really great.
Ethylene glycol serves as a solvent here. It has a very high boiling point (~200 degrees C); the fact that the reaction needs to be heated so much is why this solven tis used.
Good question. One reason I’ve read is the greater strength of the C-O (pi) bond as opposed to the C-C (pi) bond. I’m not sure this is the whole picture but it is at least a component.
Thanks for answer.
Yes, but for a Wolff-Kishner reaction that does not imply the use of a strong base (i.e. hydroxide), would water be able to deprotonate that amine in step one to give the N-N double bond? I ask because in the synthesis of the drug Amiodarone, they do not specify basic conditions. Rather, they allude that they use hydrazine hydrate only.
Water isn’t basic enough to deprotonate. The pKa of the hydrazone hydrogens is about 22, whereas that of H3O(+) is -2. The equilibrium would be 24 orders of magnitude in opposition to the desired reaction.
I found a publicly available reference to the synthesis here: : http://bit.ly/J2TCRA
Although they don’t specifiy basic conditions in the reaction scheme, it’s not uncommon to leave out details such as base, solvent and heat. The Wolff Kishner is generally a very unfavorable reaction as it is – it requires heating at 180 degrees C or more – so I am nearly 100% certain that they merely omitted writing base in the scheme here.
Great! Thank you.
does this reduce alcohols to alkanes too?
no, just ketones and aldehydes
i think it is a very good way of representing this method.i like it