Swern Oxidation of Alcohols To Aldehydes and Ketones
Description: The Swern Oxidation is a method of oxidizing primary and secondary alcohols to aldehydes and ketones, respectively. It uses oxalyl chloride (COCl)2 and dimethylsulfoxide (DMSO) with triethylamine (NEt3) as a base.

Notes:
- Oxalyl chloride (COCl)2 is used to activate dimethyl sulfoxide (CH3S(O)CH3), resulting in a reactive intermediate that reacts with the primary or secondary alcohol. In the second step, triethylamine (NEt3) is added which results in an elimination reaction giving the C=O bond.
- The reaction only works with primary and secondary alcohols. Tertiary alcohols are not affected since they do not have a C-H bond on the same carbon as the C-OH bond.
- It generates a *lot* of gas (carbon dioxide and carbon monoxide) and for this reason is generally done at low temperatures (e.g. -78°C). A notable byproduct is dimethyl sulfide, which has a rotten cabbage / egg smell.
See Also: Equivalent to oxidation with pyridinium chlorochromate (PCC) but preferable from an environmental standpoint since it does not require toxic chromium.
Examples:
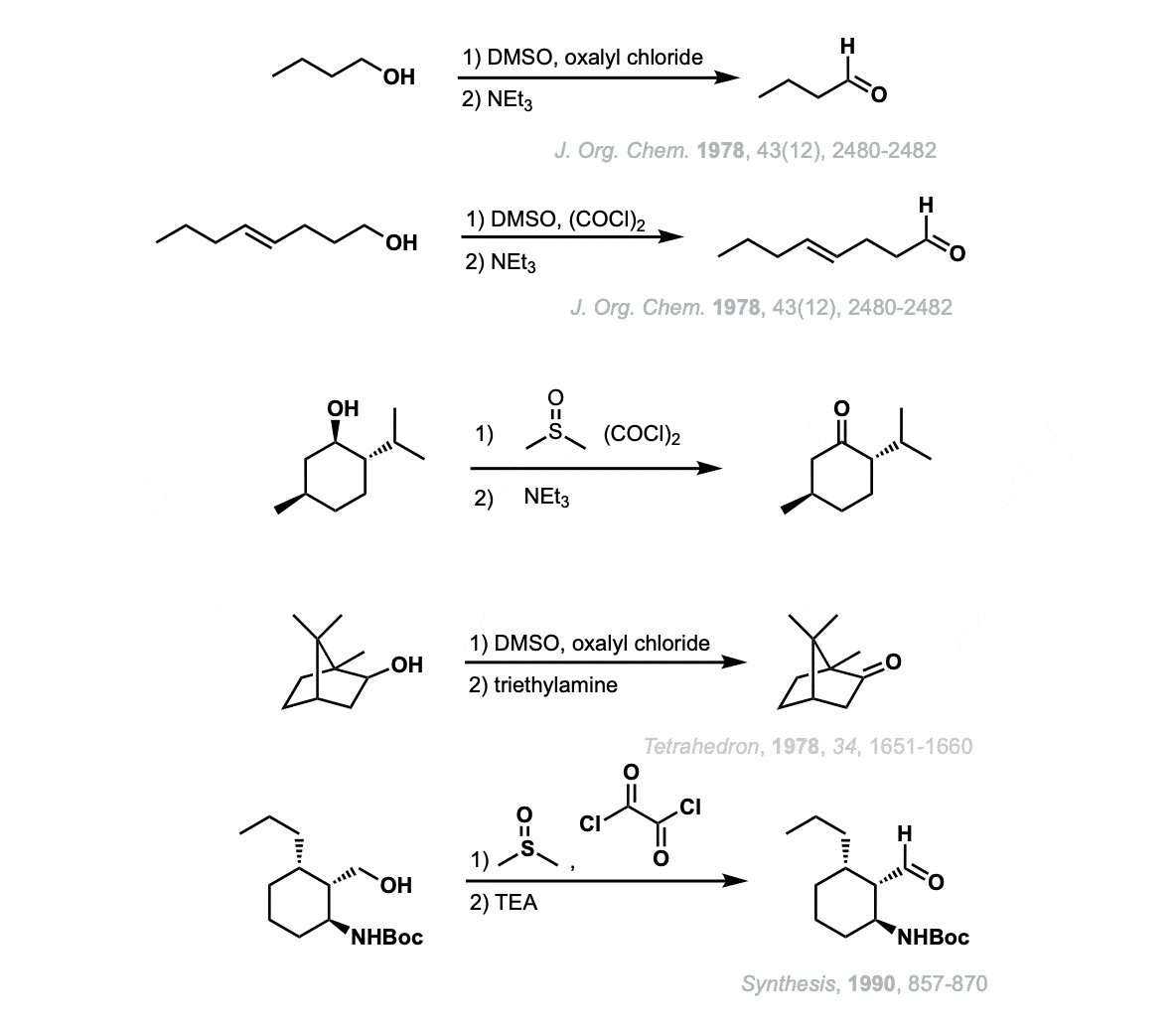
Notes:
- Examples 1 and 2 show oxidations of simple primary alcohols to give aldehydes.
- Example 3 shows the oxidation of a secondary alcohol (menthol).
- Example 4 also shows oxidation of a secondary alcohol (camphor) although drawn in perspective.
- The final example (5) shows oxidation of the primary alcohol to give an aldehyde in the presence of a protected amine.
Note that multiple equivalents of Swern reagent can be used to oxidize multiple alcohols to give carbonyls.
Tertiary alcohols are unaffected.
Mechanism:
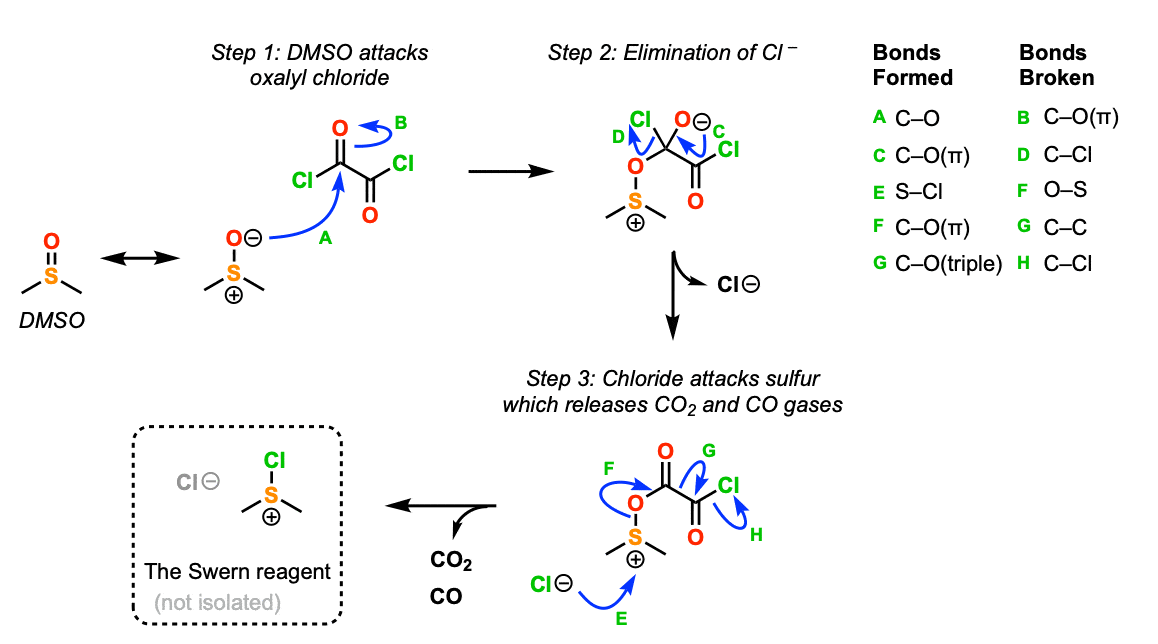
This mechanism can be divided into two parts: 1) activation of dimethyl sulfoxide, and 2) oxidation of the alcohol.
Activation of dimethyl sulfoxide is shown below. In the first step, the oxygen of DMSO acts as a nucleophile, attacking oxalyl chloride (Step 1, arrows A and B) to give a tetrahedral intermediate, which undergoes elimination (Step 2, arrows C and D) to give a nucleophilic acyl substitution product.
This product is then attacked at sulfur by chloride ion (Step 3, arrows E, F, G, H)
In the second phase of the reaction, the alcohol attacks the chlorosulfonium salt (Step 4, arrows I and J) displacing chloride ion and forming a new S-O bond. After deprotonation of the oxygen (Step 5, arrows K and L) addition of triethylamine (NEt3) results in deprotonation of one of the methyl groups adjacent to sulfur, resulting in a carbanion (Step 6, arrows M and N). This carbanion then performs an intramolecular acid-base reaction to deprotonate the C-H bond on the alcohol carbon, resulting in elimination of the sulfur species as dimethyl sulfide (Step 7, arrows O, P, Q) and formation of the new C-O pi bond in the carbonyl.
The rotten cabbage smell of dimethyl sulfide is a sign of a successful reaction! (Although experienced chemists will be able to carry out this reaction without stinking up the entire lab!).
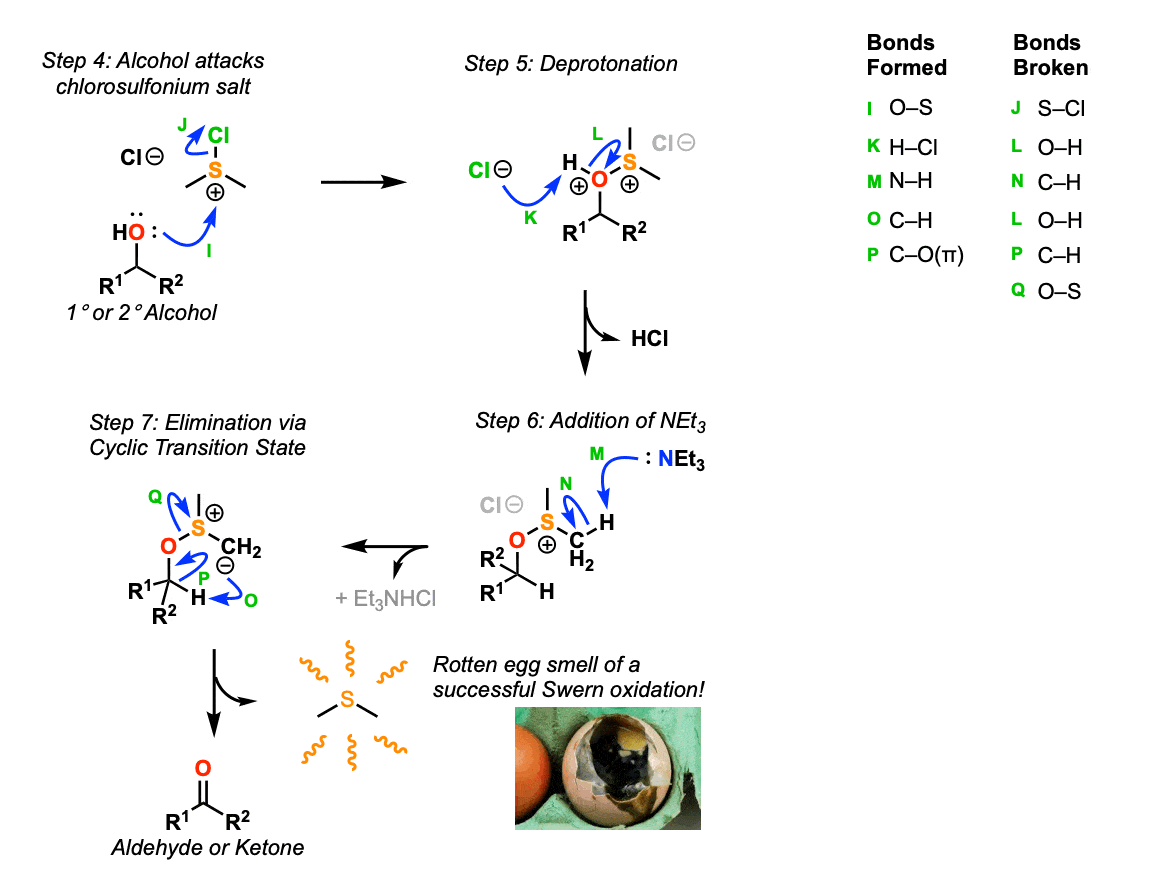
Notes:
Note that the oxidation is essentially an elimination reaction where a good leaving group has been placed on oxygen and deprotonation forms the C-O pi bond.
See Reference 4 for a procedure of how this is actually carried out in the lab. From a practical perspective, it’s important to keep the temperature low to avoid violent expulsions of gas (CO2 and CO) from the reaction mixture. Secondly, it’s important not to add NEt3 until after the alcohol has finished reacting with the chlorosulfonium salt, otherwise alkoxythiomethyl ethers will form. (RCH2OCH2SCH3).
(Advanced) References and Further Reading
- Oxidation of alcohols by “activated” dimethyl sulfoxide. A preparative, steric and mechanistic study
Omura, K.; Swern, D.
Tetrahedron 1978, 34 (11): 1651–1660
DOI: 10.1016/0040-4020(78)80197-5 - Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide “activated” by oxalyl chloride
Mancuso, A. J.; Huang, S.-L.; Swern, D.
J. Org. Chem. 1978, 43 (12): 2480–2482
DOI: 10.1021/jo00406a041 - Mechanisms of dimethylsulfoxide oxidations
Kurt Torssell
Tetrahedron Letters 1966 7 (37), 4445-4451
DOI: 10.1016/S0040-4039(00)70057-8
These papers are on what is now commonly called the “Swern oxidation” after its developer, Daniel Swern. This method is rather mild and uses DMSO, a common solvent, as the oxidant. However, this also results in the formation of dimethyl sulfide (which is notoriously stinky) as the product of the reaction, one of its noteworthy characteristics. - SYNTHESIS OF 1,1-DIMETHYLETHYL (S)-4-FORMYL-2,2-DIMETHYL-3-OXAZOLIDINECARBOXYLATE BY OXIDATION OF THE ALCOHOL
Alessandro Dondoni and Daniela Perrone
Org. Synth. 2000, 77, 64
DOI: 10.15227/orgsyn.077.0064
The final step (4 -> 5) in this multistep procedure is a Swern oxidation. This is from Organic Syntheses, a source of reliable, independently tested synthetic organic procedures