Epoxides From Addition of Sulfur Ylides To Aldehydes and Ketones (Corey-Chaykovsky)
Description: Sulfur ylides add to carbonyls (aldehydes and ketones) to form epoxides, releasing dimethyl sulfide. (This is sometimes called the Corey-Chaykovsky reaction).
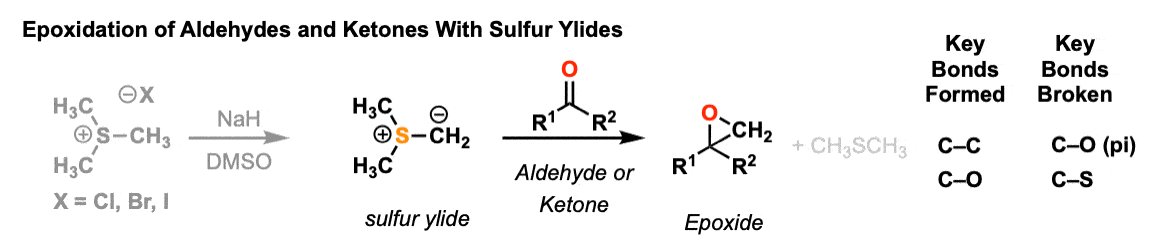
Notes: The ylide is made through deprotonation of a sulfonium salt (e.g. Me3S(+) ) with a strong base such as sodium hydride. You may recall seeing phosphorus ylides in the Wittig reaction. (A ylide is a species with adjacent opposite charges).
See Also: The second step is somewhat similar to the formation of epoxides from halohydrins. (See formation of epoxides from halohydrins)
Examples:
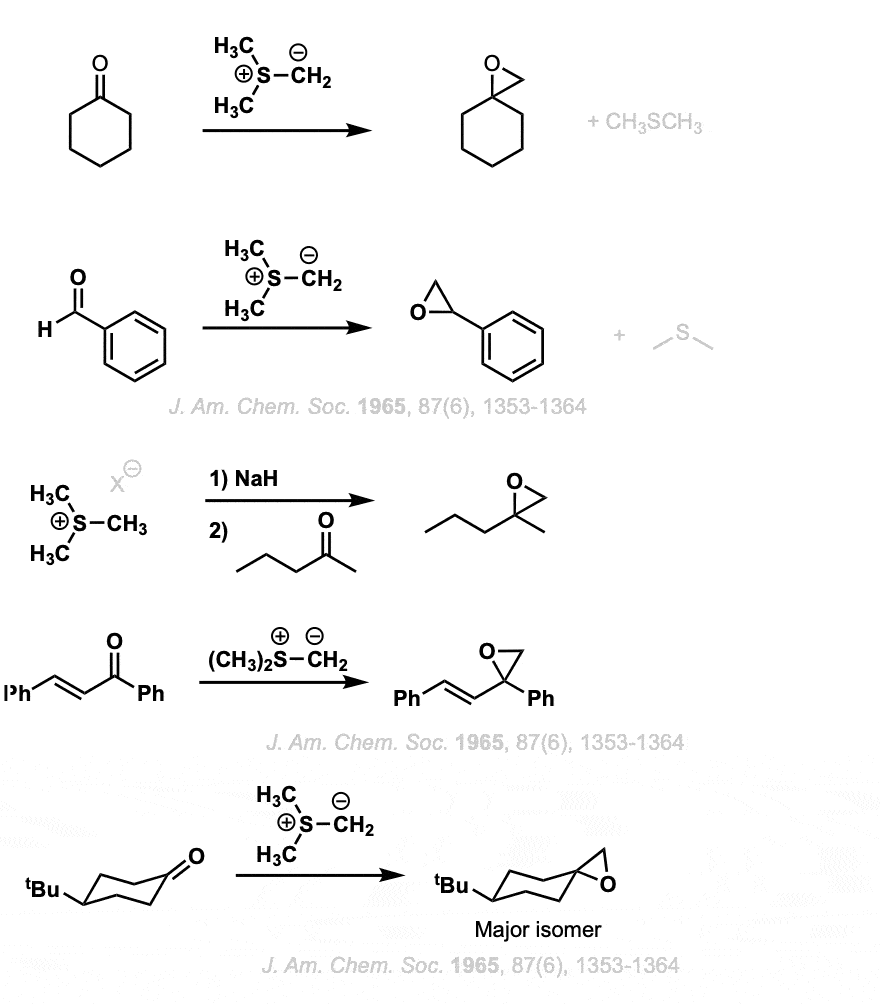
Notes:
In the first example, cyclohexanone is converted into an epoxide, and the second example shows conversion of an aldehyde into an epoxide.
With alpha, beta unsaturated ketones such as in example 4, addition still occurs to the carbonyl carbon. (There is a different ylide, the sulfoxonium ylides, that will perform cyclopropanation reactions.).
Mechanism:
In the first step of this reaction, the sulfonium salt is deprotonated with NaH to give the sulfur ylide (Step 1, arrows A and B). In the second step, the carbon nucleophile attacks the carbonyl carbon, forming a new C-C bond and breaking C-O (pi) (Step 2, arrows C and D).
In the third step, the resulting alkoxide ion then attacks the adjacent carbon in an SN2 reaction, forming an epoxide and displacing dimethylsulfide as a leaving group.
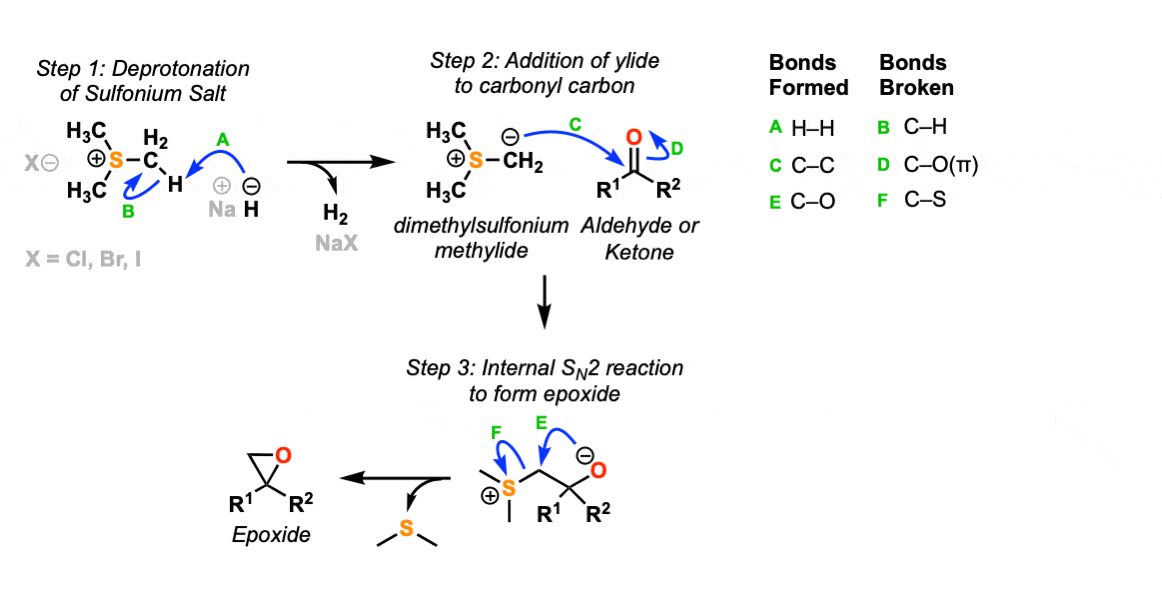
Notes: The counter-ion for the sulfonium salt can be Cl, Br, or I. Other bases can be used.
The third step is very similar to epoxide formation from a halohydrin, except the leaving group is CH3SCH3 instead of a halide ion.
(Advanced) References and Further Reading
- Twenty-five years of dimethylsulfoxonium ethylide (Corey’s reagent)
Yu.G. Gololobov, A.N. Nesmeyanov, V.P. lysenko, I.E. Boldeskul
Tetrahedron, 1987, 43(12), 2609-2651
DOI: 10.1016/S0040-4020(01)86869-1
- The Chemistry of Ylids. VI. Dimethylsulfonium Fluorenylide—A Synthesis of Epoxides
A. William Johnson and Robert B. LaCount
Journal of the American Chemical Society 1961 83 (2), 417-423
DOI: 10.1021/ja01463a040 - Dimethyloxosulfonium Methylide ((CH3)2SOCH2) and Dimethylsulfonium Methylide ((CH3)2SCH2). Formation and Application to Organic Synthesis
E. J. Corey and Michael Chaykovsky
Journal of the American Chemical Society 1965 87 (6), 1353-1364
DOI: 10.1021/ja01084a034