Acidic Hydrolysis of Amides
Description: Treatment of amides with aqueous acid (and heat) gives carboxylic acids.
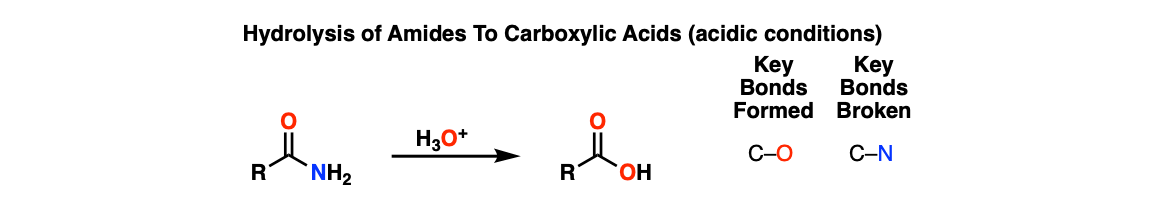
Notes: The vague, “H3O+” is shown here. In practice this procedure may involve aqueous HCl or H2SO4. Here is a link to a procedure.
Examples:
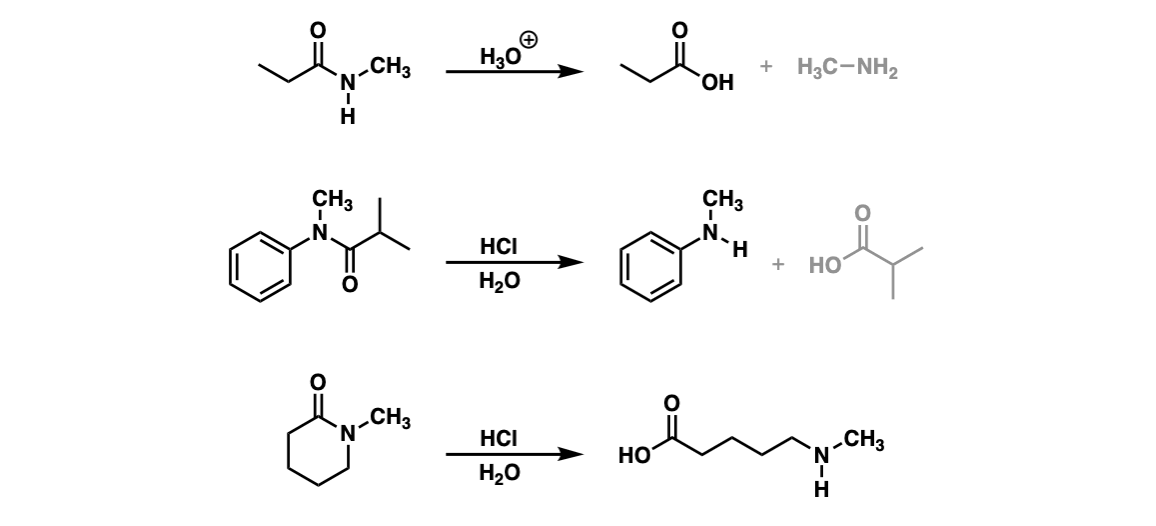
Notes: Example 1 shows the hydrolysis of a secondary amide with acid to give a carboxylic acid. Example two shows the hydrolysis of a tertiary amide to give a carboxylic acid and an amine. The third example shows the hydrolysis of a cyclic amide (a “lactam”) to give a linear product.
Mechanism:
This is a reaction mechanism that follows the classic “PADPED” pattern. Protonation of the oxygen (Step 1, arrows A and B) results in a charged species that undergoes addition by nucleophilic water (Step 2, arrows C and D). This is followed by proton transfer (Steps 3 and 4, arrows E and F) to NH2, giving a better leaving group NH3+ which then undergoes elimination (Step 5, arrows G and H) to give a protonated carboxylic acid which is then deprotonated by base (Step 6, arrows I and J).
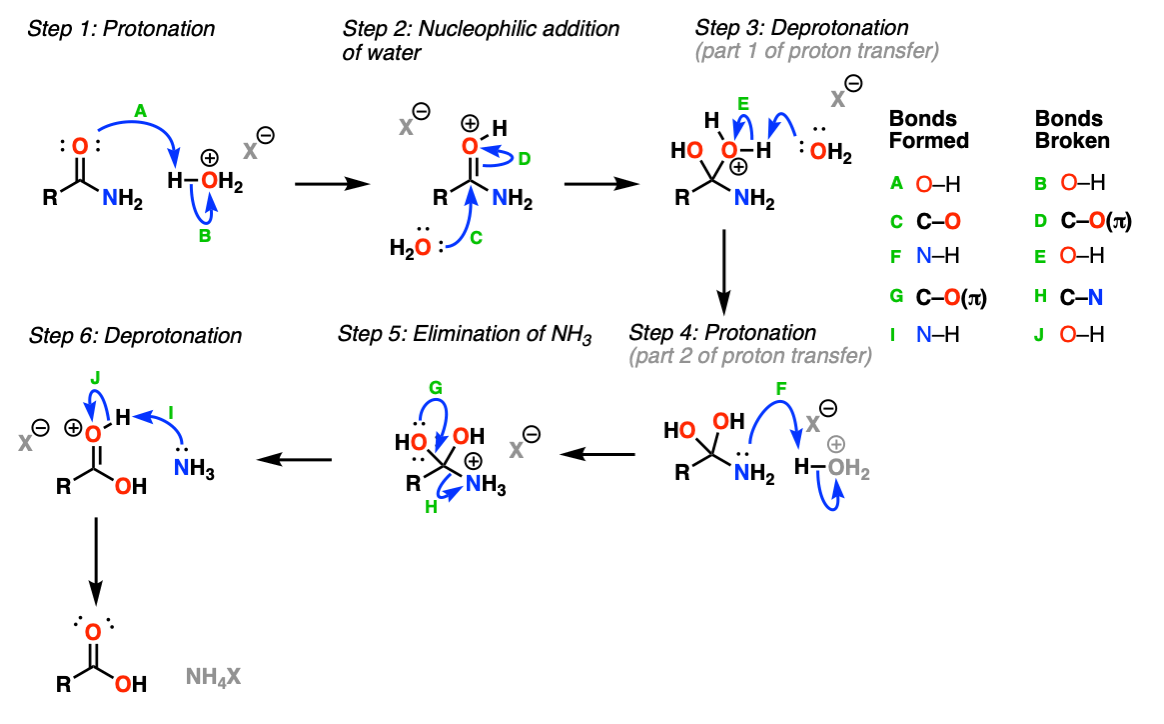
Notes: “PADPED” stands for Protonation, Addition, Deprotonation/Protonation, Elimination, Deprotonation. The same
H3O+ is shown as the acid here, which can be formed through combination of HCl + H2O or H2SO4 + H2O among many other combinations.
Real-Life Examples:
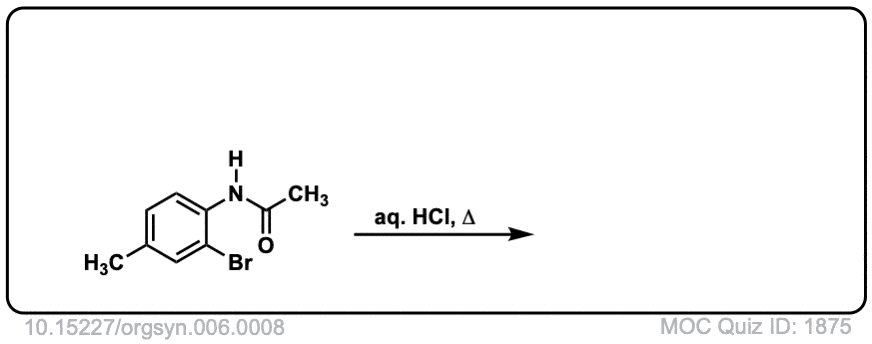
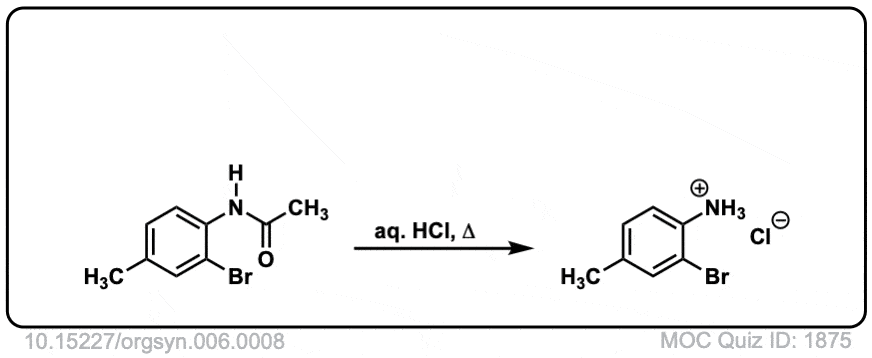
Org. Synth. 1926, 6, 8
DOI Link: 10.15227/orgsyn.006.0008
 Click to Flip
Click to Flip
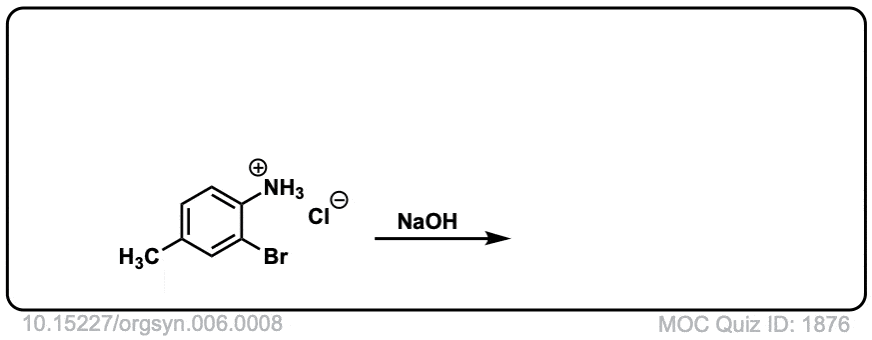
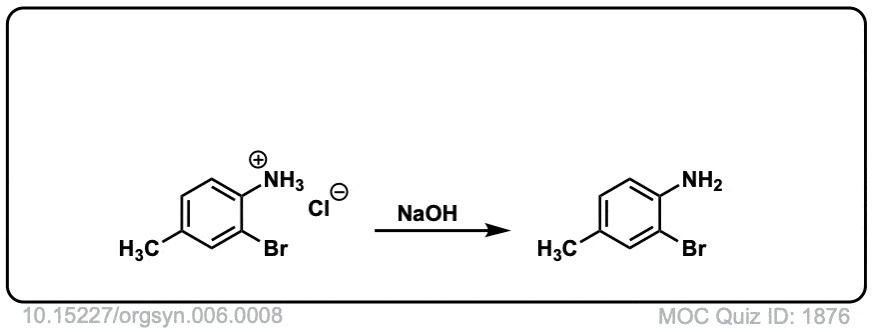
Org. Synth. 1926, 6, 8
DOI Link: 10.15227/orgsyn.006.0008
 Click to Flip
Click to Flip
Org. Synth. 1937, 17, 7
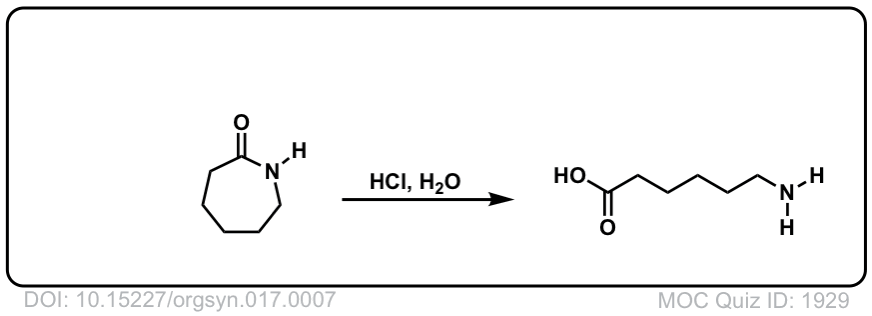
DOI Link: 10.15227/orgsyn.017.0007
 Click to Flip
Click to Flip
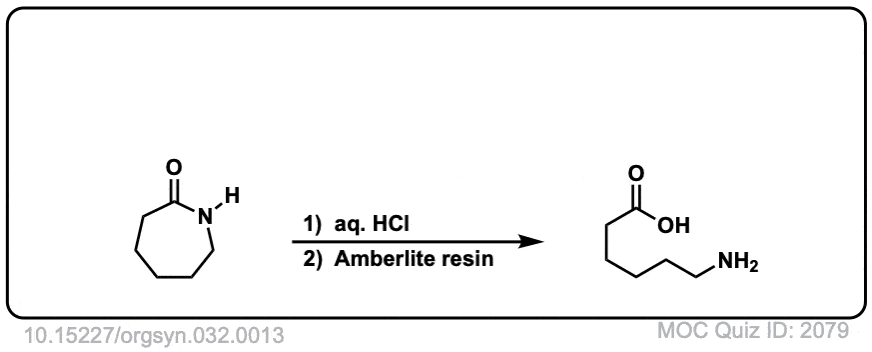
Org. Synth. 1952, 32, 13
DOI Link: 10.15227/orgsyn.032.0013
 Click to Flip
Click to Flip