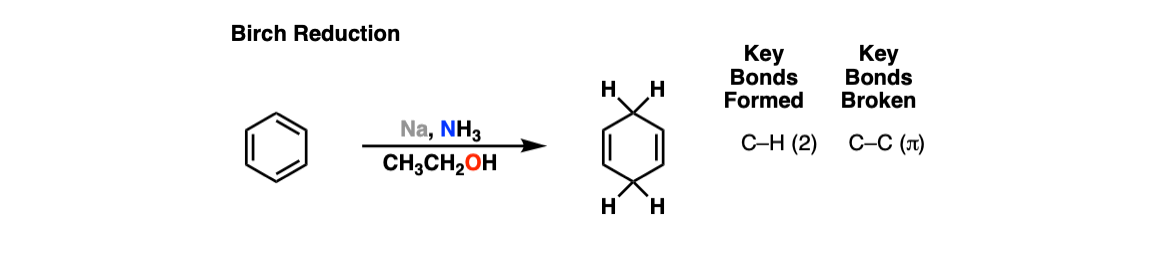
Birch Reduction of Aromatic Rings
Description: The Birch reduction is the reduction of an aromatic ring with sodium (or potassium) metal in ammonia (NH3) in the presence of an alcohol. The result is a 1,4-cyclohexadiene.
Notes: Don’t confuse this with NaNH2/NH3, which is formation of a benzyne.
The substitution pattern of 1,4-cyclohexadiene product depends on whether the ring is attached to an electron-withdrawing group (EWG) or an electron donating group (EDG).
Examples:
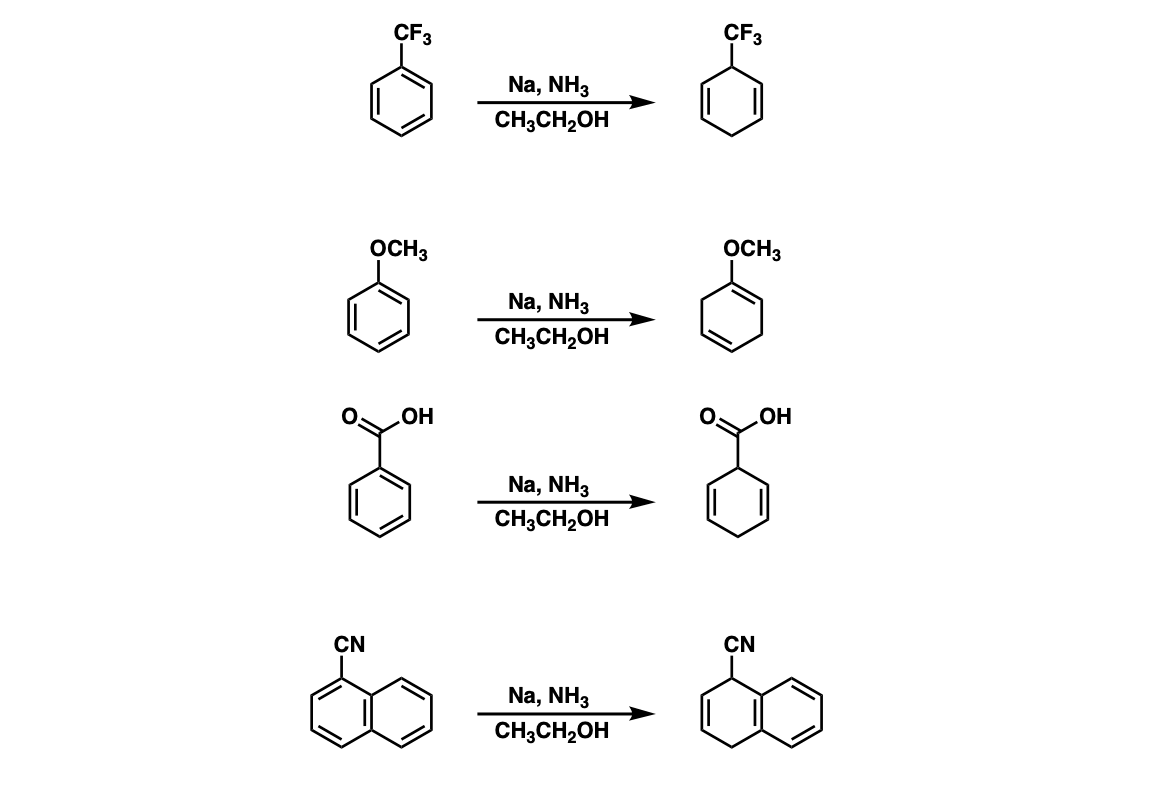
Notes: The presence of an electron-withdrawing group tends to stabilize an anion directly adjacent to it, resulting in patterns like those seen in examples 1, 3, and 4.
With an electron-donating group (such as OCH3) the intermediate anion is formed as far away as possible, resulting in the formation of an enol ether in conjugation with the new double bond.
For more on this, see: Birch Reduction of Aromatic Rings
Mechanism:
The role of ammonia as solvent is to dissolve the sodium metal, which ends up forming a deep blue solution. The active reagent in the Birch reduction is a free electron, solvated by NH3, which is responsible for the strong color.
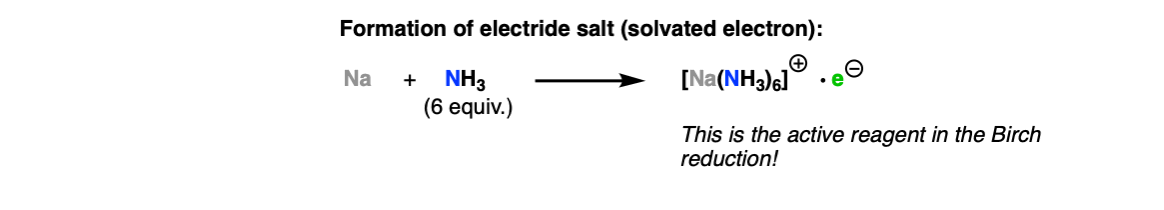
The first step is addition of the electron to the aromatic ring (Step 1) leading to the formation of a “radical anion”. In the next step, the carbanion is protonated by the alcohol (Step 2, arrows A and B) forming a new C-H bond and leaving behind a free radical. The radical is reduced to another carbanion (Step 3), which is then protonated again (Step 4, arrows C and D) giving the 1,4 cyclohexadiene product.
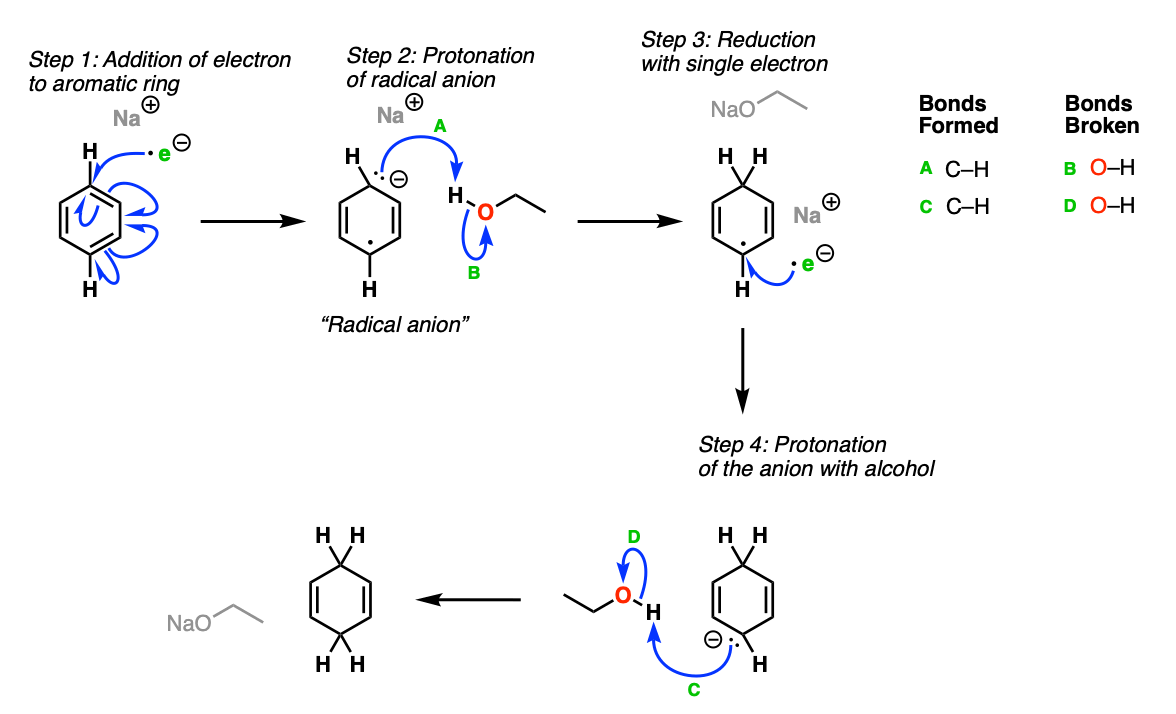
Notes: One can also just draw the electron as being attached to the sodium metal in the beginning, such as Na• .
The purpose of the alcohol (ROH) is to protonate the anion at the end. In the absence of alcohol, undesired C-C bond formation tends to occur.
Real-Life Examples:
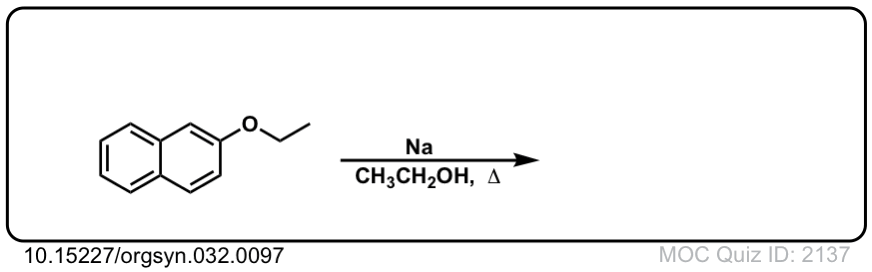
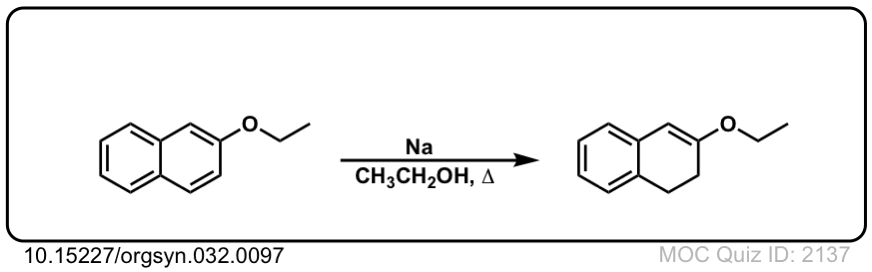
Org. Synth. 1952, 32, 97
DOI Link: 10.15227/orgsyn.032.0097
 Click to Flip
Click to Flip
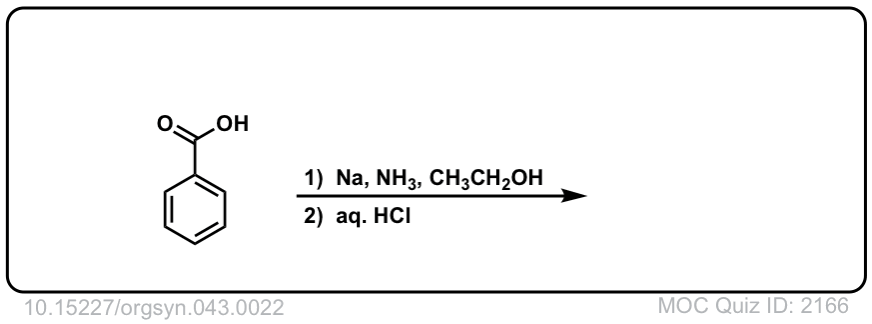
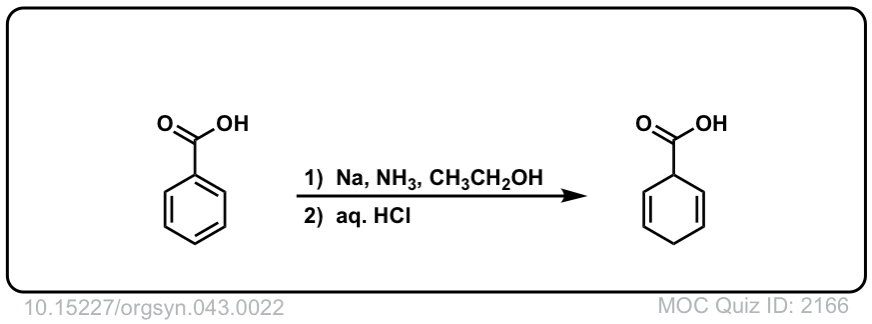
Org. Synth. 1963, 43, 22
DOI Link: 10.15227/orgsyn.043.0022
 Click to Flip
Click to Flip
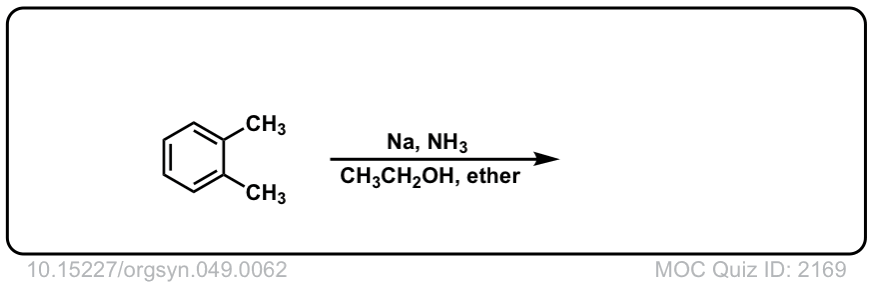
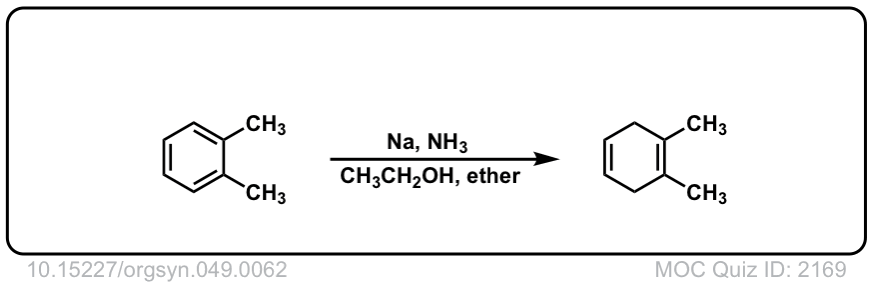
Org. Synth. 1969, 49, 62
DOI Link: 10.15227/orgsyn.049.0062
 Click to Flip
Click to Flip
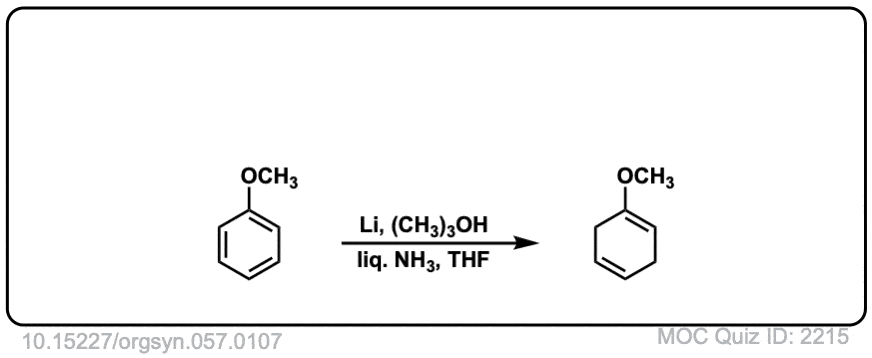
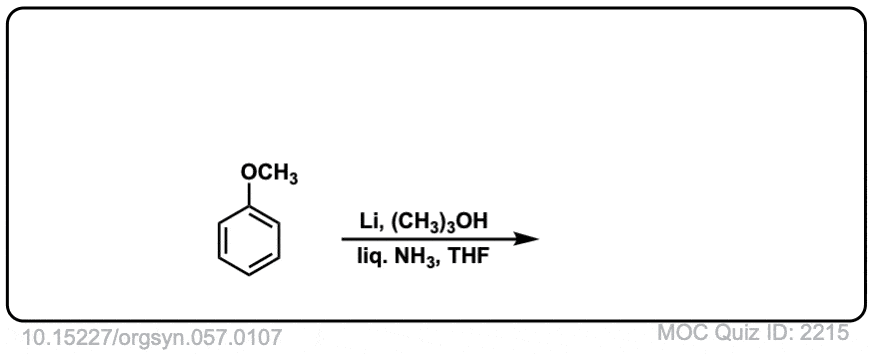
Org. Synth. 1977, 57, 107
DOI Link: 10.15227/orgsyn.057.0107
 Click to Flip
Click to Flip