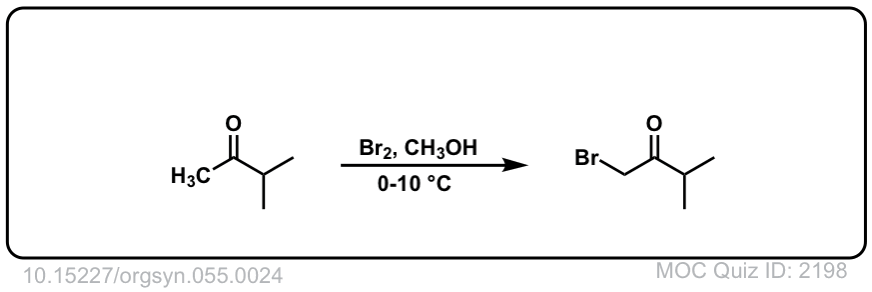
Halogenation Of Ketones via Enols
Description: Treatment of ketones with bromine (Br2) in the presence of acid will result in formation of a new C-Br bond at the “alpha” position.
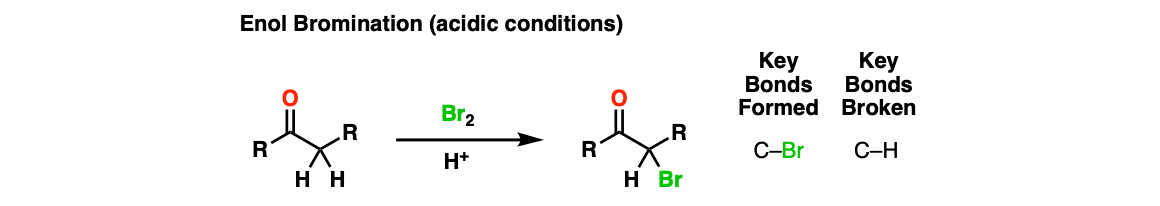
Notes: The purpose of the acid is to catalyze formation of the enol from the ketone, which is the active nucleophile in the reaction. Instead of Br2, one can use a similar source of electrophilic bromine like N-bromosuccinimide (NBS). Various acids can be used (e.g. HBr, acetic acid (AcOH).
Examples:
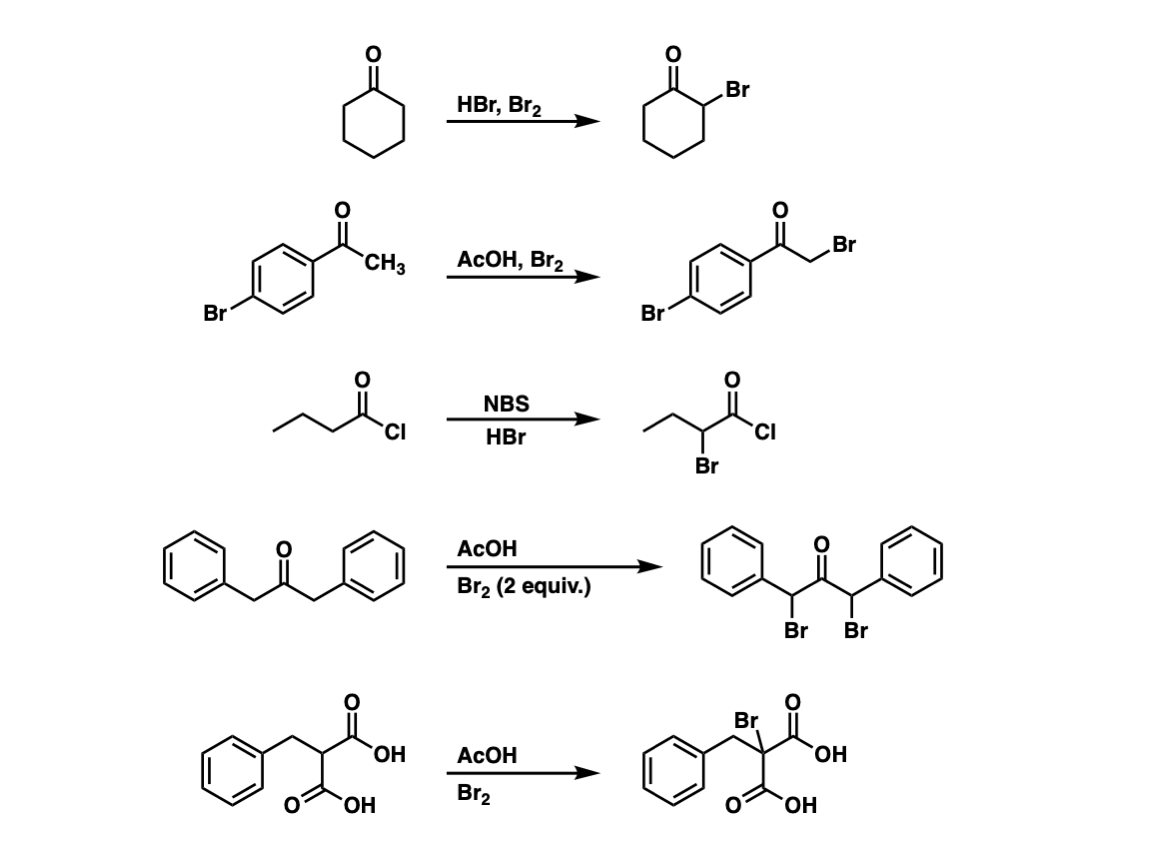
Notes: Example 1 shows formation of a new C-Br bond using HBr and Br2. Example 2 shows the use of acetic acid (AcOH). Example 3 shows how this reaction can occur on acid halides (this is a key step in the Hell-Vollhard-Zelinsky reaction).
Mechanism:
The reaction begins with protonation of the carbonyl oxygen with acid (Step 1, arrows A and B) which accelerates keto-enol tautomerism. Deprotonation of the C-H bond on the alpha-carbon (Step 2, arrows C , D, and E) results in the formation of the enol, which is the active nucleophile in this reaction. The nucleophilic enol carbon then reacts with the excellent electrophile, bromine (Step 3, arrows F, G, H) resulting in a new C-Br bond. The carbonyl oxygen is then deprotonated with weak base (Step 4, arrows I and J) to give the alpha-bromo ketone.
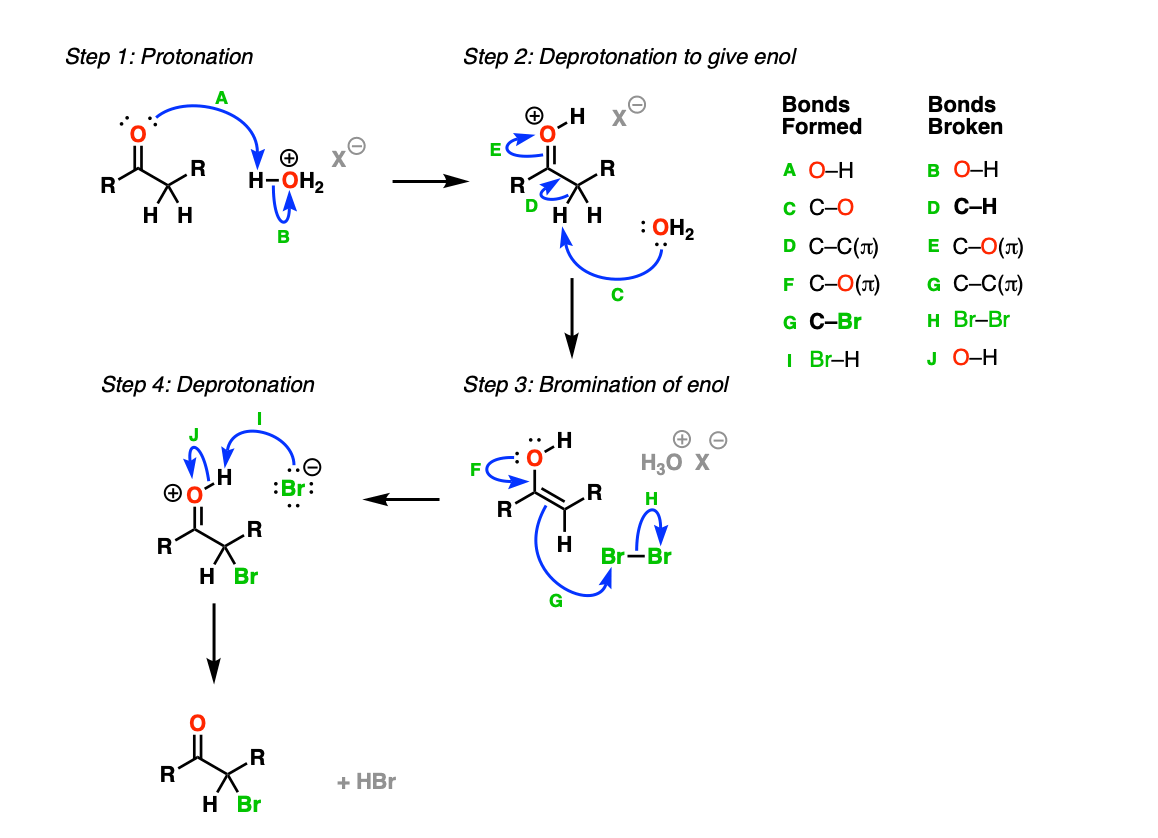
Notes: The key step here is step 3, where the enol acts as a nucleophile and attacks Br2, forming the new C-Br bond.
Real-Life Examples:
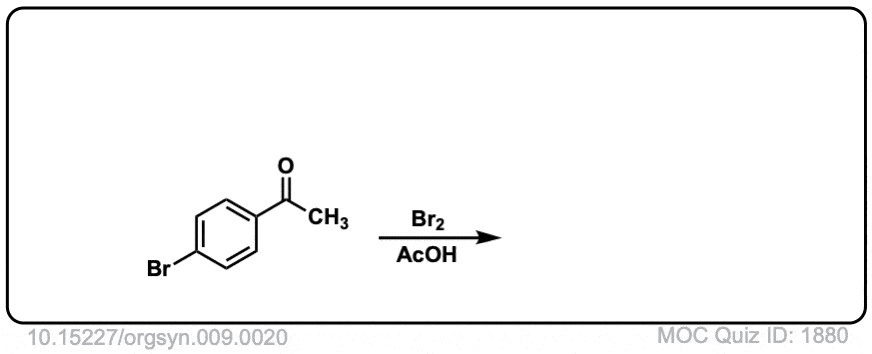
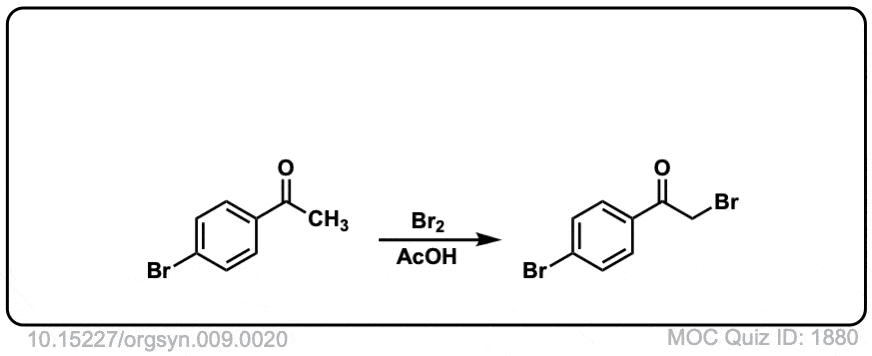
Org. Synth. 1929, 9, 20
DOI Link: 10.15227/orgsyn.009.0020
 Click to Flip
Click to Flip
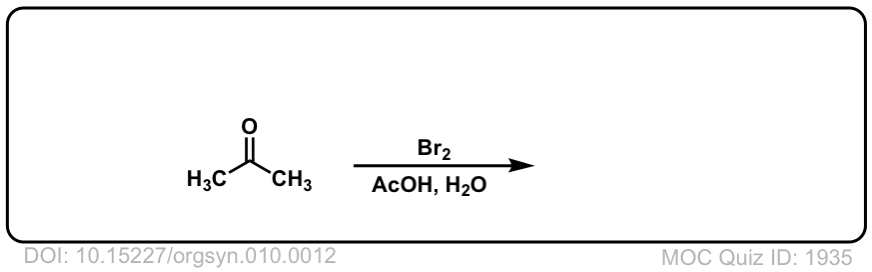
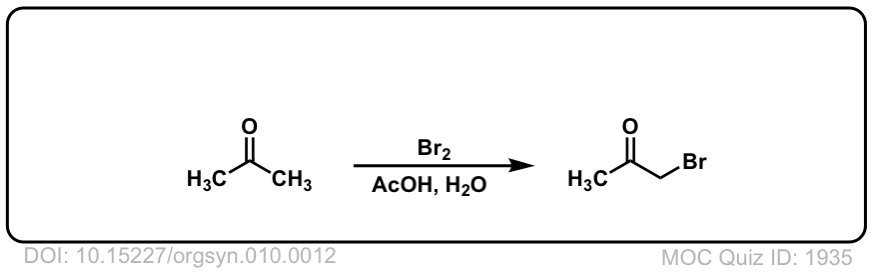
Org. Synth. 1930, 10, 12
DOI Link: 10.15227/orgsyn.010.0012
 Click to Flip
Click to Flip
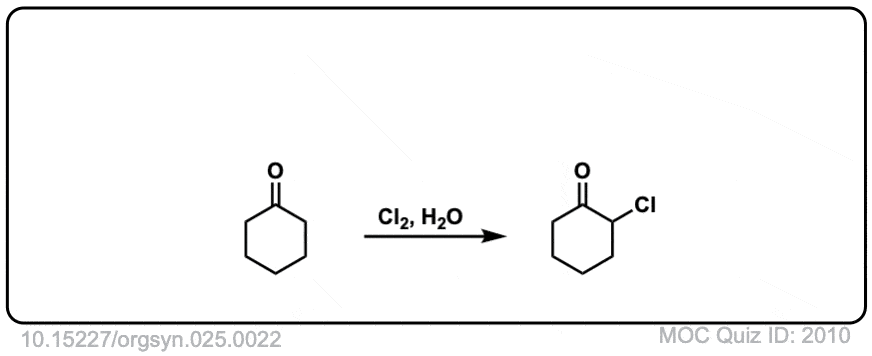
Org. Synth. 1945, 25, 22
DOI Link: 10.15227/orgsyn.025.0022
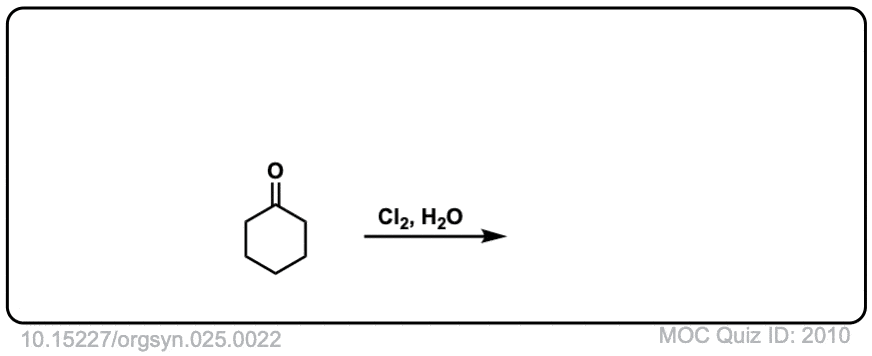 Click to Flip
Click to Flip
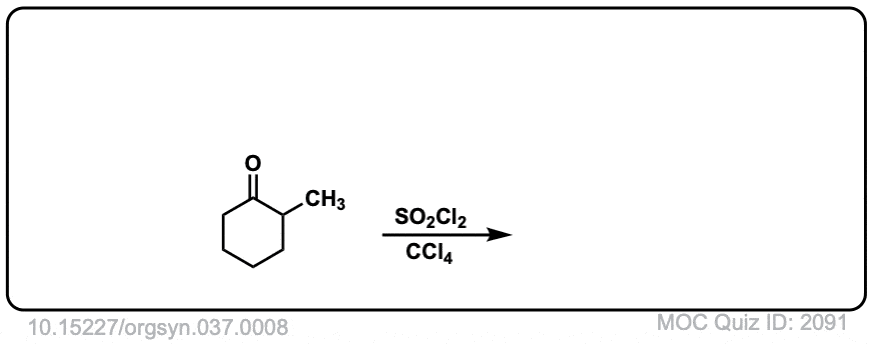
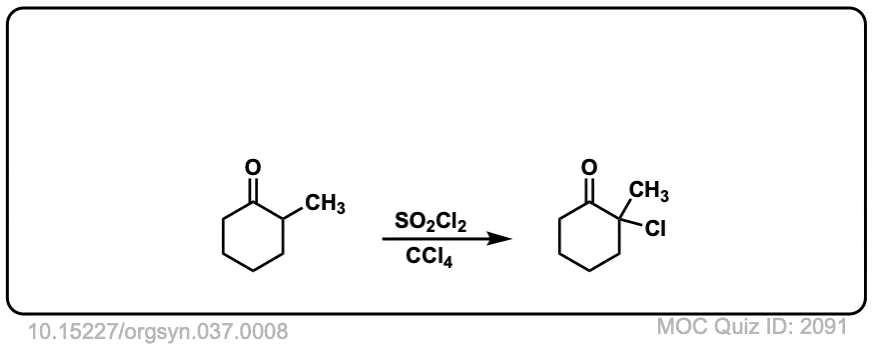
Org. Synth. 1957, 37, 8
DOI Link: 10.15227/orgsyn.037.0008
 Click to Flip
Click to Flip
Org. Synth. 1967, 47, 62
DOI Link: 10.15227/orgsyn.047.0062
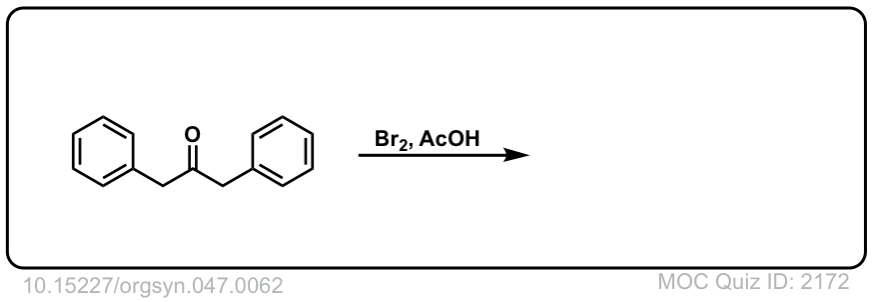 Click to Flip
Click to Flip
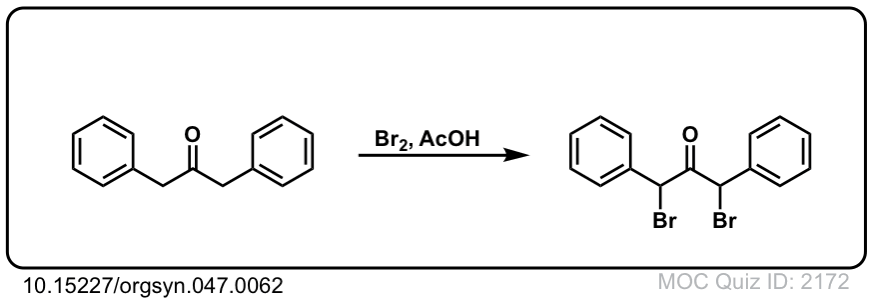
Org. Synth. 1976, 55, 27
DOI Link: 10.15227/orgsyn.055.0027
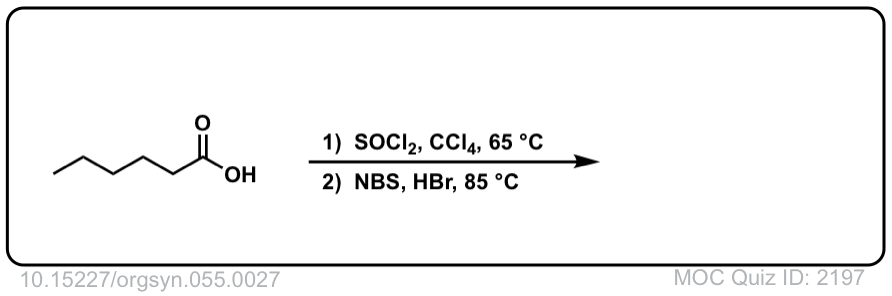 Click to Flip
Click to Flip
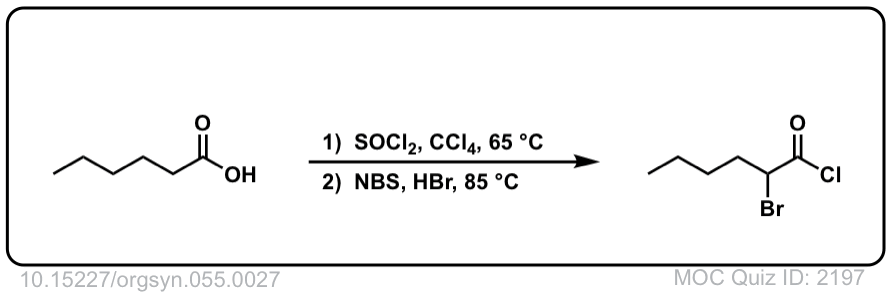
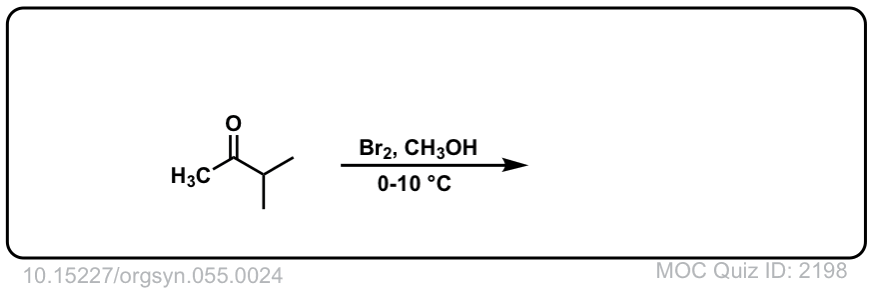
Org. Synth. 1976, 55, 24
DOI Link: 10.15227/orgsyn.055.0024
 Click to Flip
Click to Flip