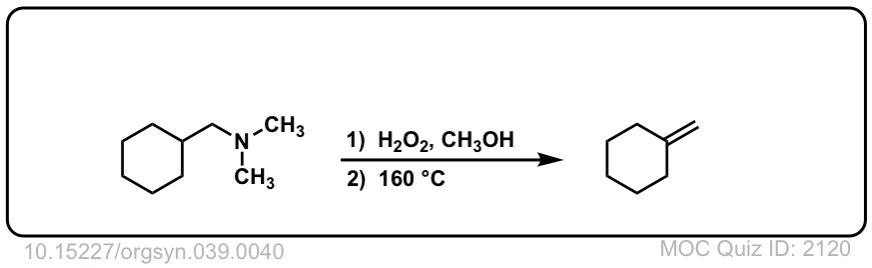
Cope Elimination
Description: The Cope elimination is a two step process whereby a tertiary amine is 1) oxidized to a N-oxide with an oxidant like H2O2, and then 2) heated to give an elimination product in a concerted reaction.
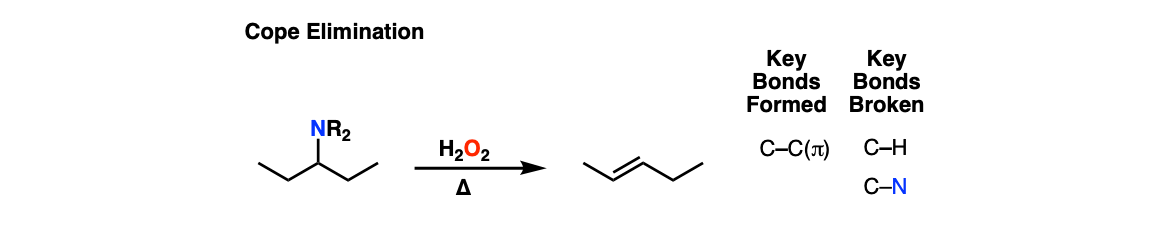
Notes: Many other oxidants can be used (e.g. m-CPBA). The amine has to be tertiary, otherwise undesired side-reactions will occur.
Examples:
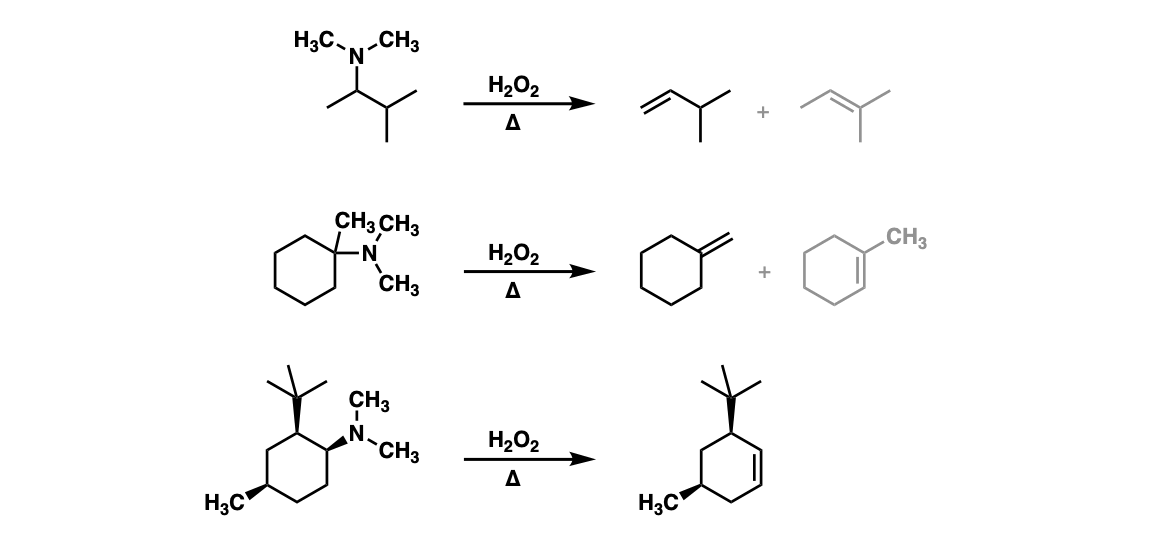
Notes: Example 1 shows an elimination where either a mono-substituted or tri-substituted alkene can occur. The reaction tends to favor formation of the less-substituted alkene.
Example 2 shows the preferential formation of a disubstituted alkene vs a trisubstituted alkene.
Example 3 shows the importance of stereochemistry. It’s important that the hydrogen that is removed in the Cope elimination is on the same side of the cyclohexane ring as the N-oxide. Formation of the Zaitsev alkene in this case is impossible.
Mechanism:
In the first step the amine attacks hydrogen peroxide (Step 1, arrows A and B) resulting in a protonated N-oxide, which is then deprotonated by the resulting hydroxide ion (Step 2, arrows C and D). Upon heating, a concerted elimination occurs (Step 3, arrows E, F and G) resulting in the formation of the new alkene.
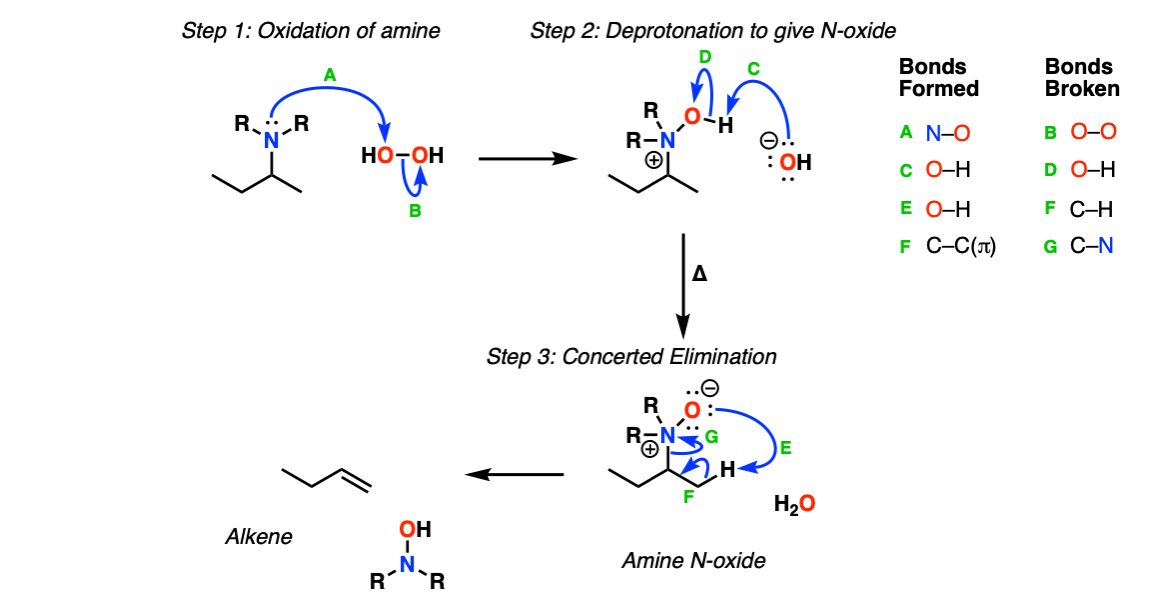
Notes: The elimination is a concerted, six-electron pericyclic transition state (much like the transition state for other pericyclic reactions like the Cope, Claisen and Diels-Alder reactions).
See also: The Cope Elimination
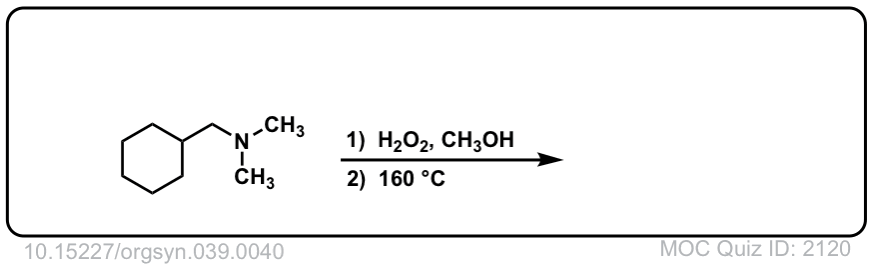
Real-Life Example:
Org. Synth. 1959, 39, 40
DOI Link: 10.15227/orgsyn.039.0040
 Click to Flip
Click to Flip