Formation of Amides From Esters
Description: Treatment of esters with amines and heat results in an amide.
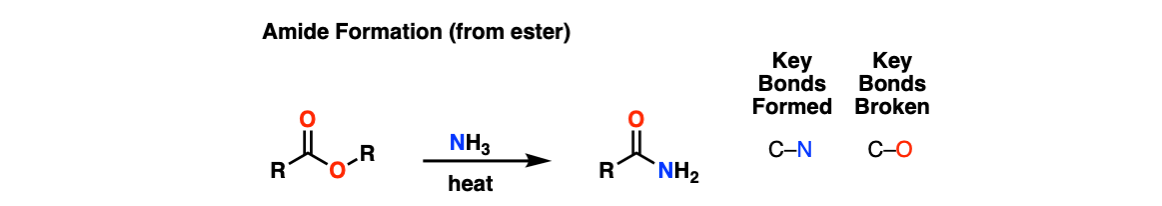
Notes: This can be used to form primary, secondary, and tertiary amides.
Examples:
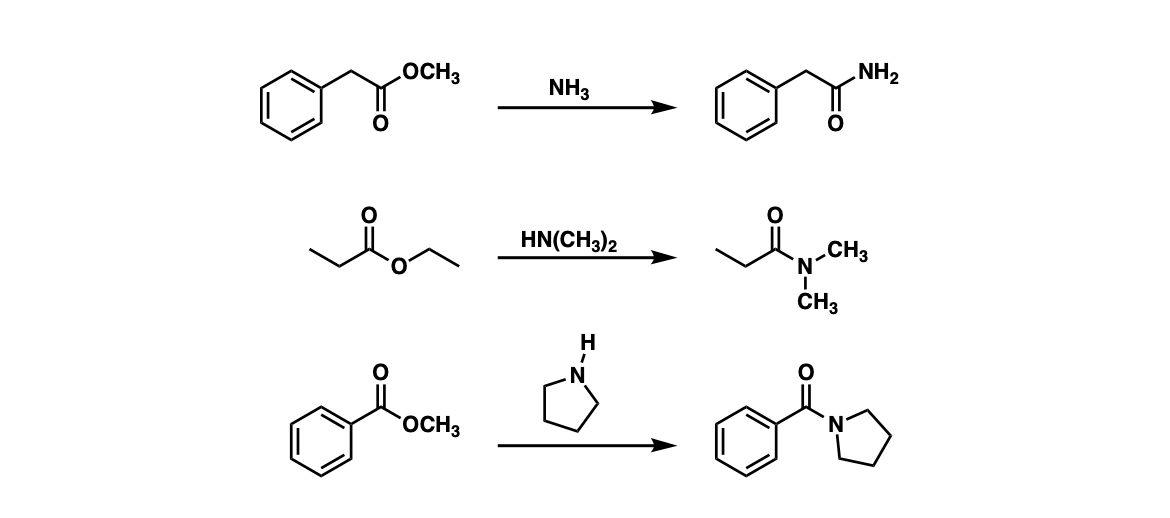
Notes: Example 1 shows the formation of a primary amide through the reaction of an ester with ammonia (NH3). Examples 2 and 3 show the formation of tertiary amides through treating esters with secondary amines.
Mechanism:
The first step is addition of NH3 to the carbonyl (Step 1, arrows A and B) to form a tetrahedral intermediate. This is followed by elimination of an alkoxide (Step 2, arrows C and D) to give a protonated amide, which is then deprotonated (Step 3, arrows E and F) to give the amide.
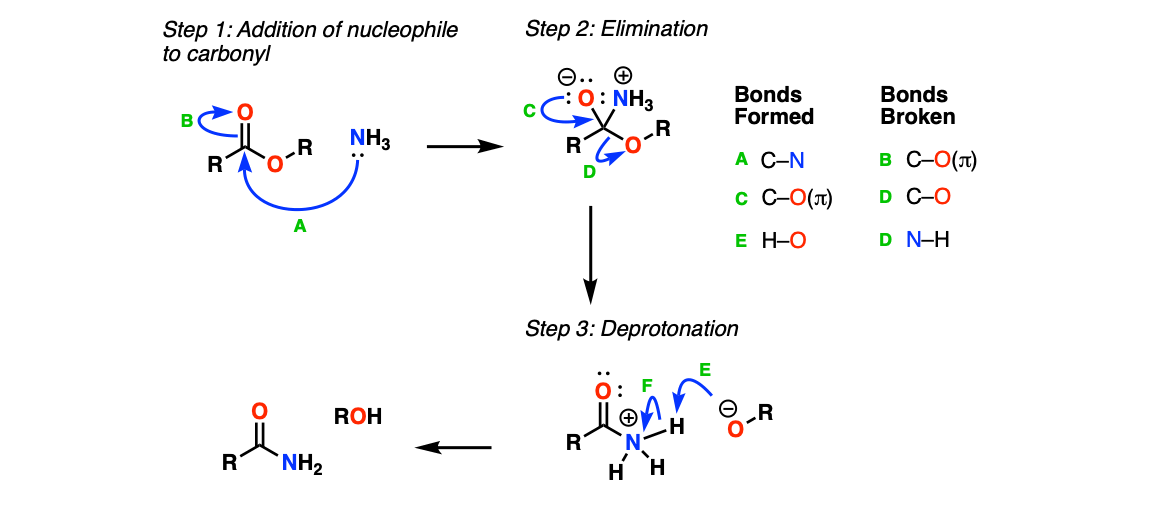
Notes: Step 2 has been simplified a bit. It’s probably a bit more correct to show a proton transfer between the NH3+ and the O- to give an OH, followed by the lone pair from nitrogen performing the elimination of the RO(-), and then deprotonation / tautomerization to give the neutral amide. I’ve tried to keep things very straightforward here.