Mitsunobu Reaction
Description: The Mitsunobu Reaction is a useful method for inverting the stereochemistry of an alcohol, using the reagents triphenylphosphine (PPh3), diethyl azodicarboxylate (DEAD) and a nucleophile (often a carboxylic acid).
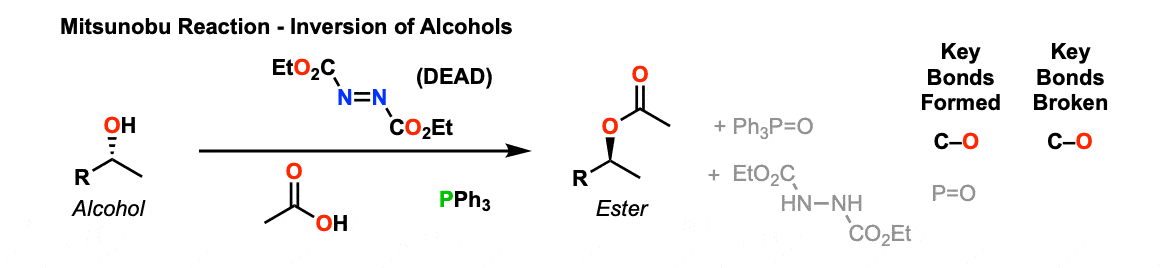
Notes: The nucleophile is often a carboxylic acid, but can also be other species so long as they have a pKa of around 13 or below. Thiols, phenols, HN3 and other species have all been used as nucleophiles. Normal alcohols (pKa 14-17) cannot.
The final product here is an ester. The ester is easily converted to an alcohol by treatment with base (see Saponification of Esters).
Thus, this is a useful method for inverting the stereochemistry of an alcohol.
See Also: The Mitsunobu reaction is generally a very reliable way of performing SN2 reactions on secondary carbons, especially when compared to converting an alcohol to a tosylate or mesylate and then displacing via an SN2 reaction.
Examples:
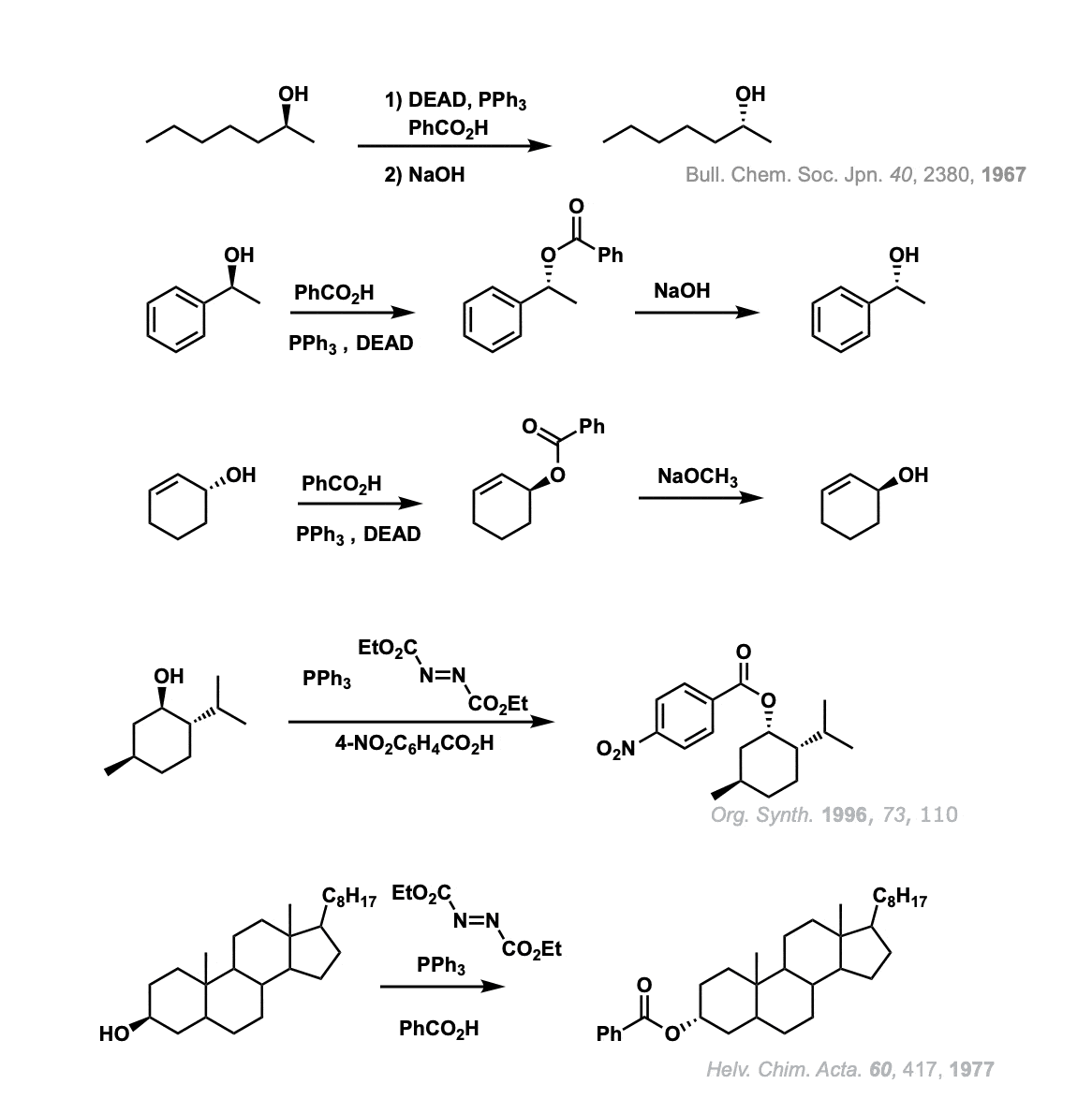
Notes:
DEAD stands for diethyl azodicarboxylate. It is necessary for the reaction to occur, as is PPh3.
In the first example, an alcohol is converted to an ester with inversion of configuration. Addition of base (NaOH) converts the ester to an alcohol.
The second example is similar, but shows the intermediate ester.
The third example uses NaOCH3 instead of NaOH with the same result. The fourth example shows inversion of configuration on the alcohol menthol (for our purposes, there’s no functional difference in using p-NO2 benzoic acid here). The last example shows the Mitsonobu reaction on cholesterol.
Mechanism:
- In this mechanism, first step is addition of Ph3P (a good nucleophile) to one of the nitrogens of DEAD (diethylazodicarboxylate) breaking a N=N bond and forming a resonance-stabilized anion (Step 1, arrows A and B).
- Next, the negatively charged nitrogen is protonated by the carboxylic acid (Step 2, arrows C and D, form N-H and break O-H) to give the product with the phosphonium ion.
- In the third step, the alcohol attacks the positively charged phosphorus and displaces the resonance stabilized anion as a leaving group. (Step 3, arrows E and F, form O-P, break P-N)
- In the fourth step, the alcohol is deprotonated by the negatively charged nitrogen (Step 4, arrows G and H, form N-H and break O-H)
- The fifth and final step is when the inversion occurs. The carboxylate anion, which was deprotonated in step 2, acts as a nucleophile in an SN2 reaction, forming a new C-O bond. The C-O bond breaks and the final product is Ph3P=O.
Afterwards, the ester product can be converted back into an alcohol by treating the product with a strong base like NaOH or NaOCH3 which results in net inversion of configuration of an alcohol.
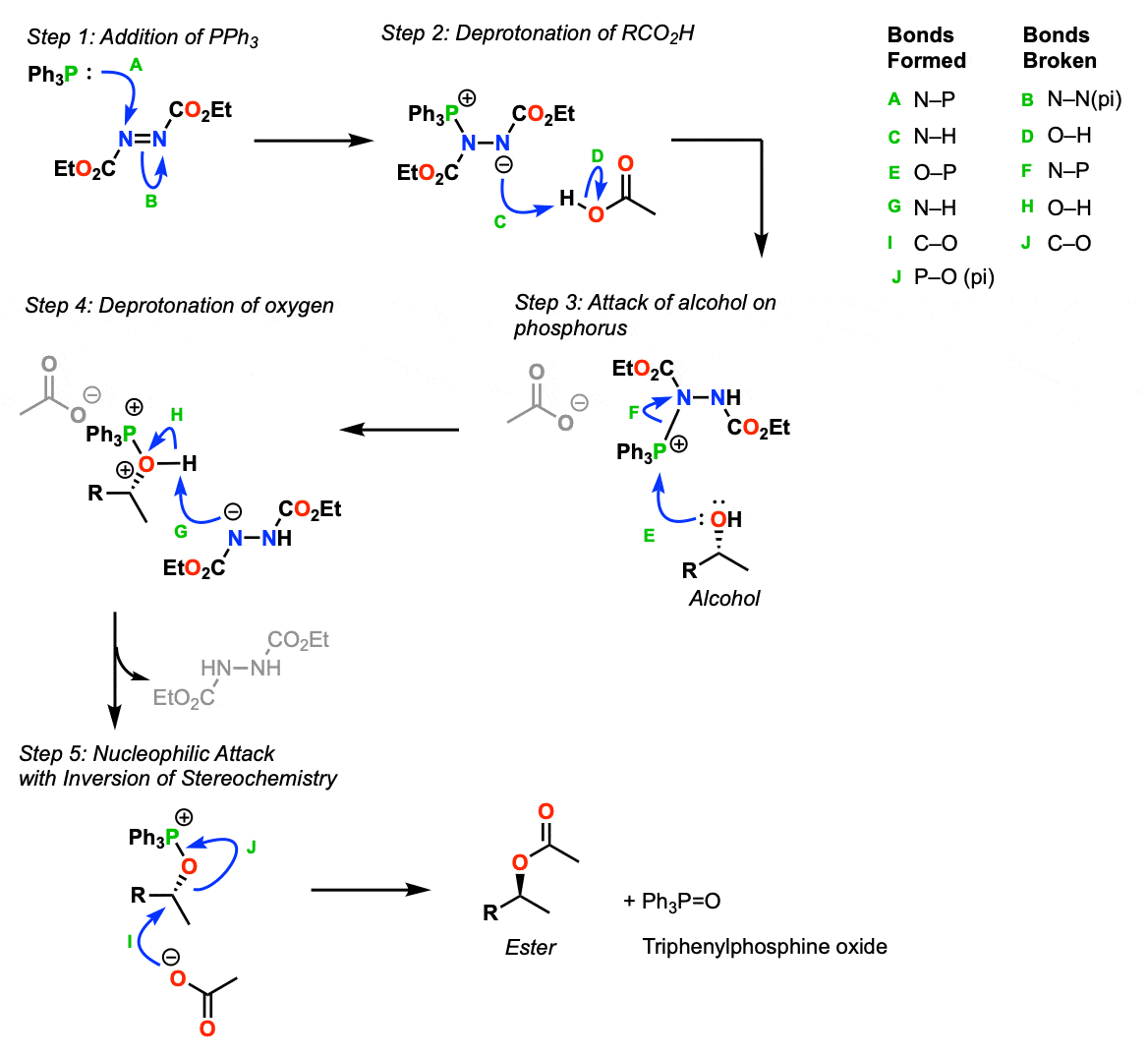
Notes: The key to the reaction is that the negatively charged hydrazide (in step 2), a weak base, is able to deprotonate the nucleophile (in this case a carboxylic acid). Otherwise the SN2 reaction in step 5 will not work because the nucleophile will not be strong enough. Alcohols do not generally work in the Mitsunobu (except for phenols pKa = 10) because they cannot be deprotonated by this base.
The driving force for this reaction is formation of the strong P=O bond.
Note that in Step 4 there are two adjacent positive charges (one on oxygen, one on phosphorus) but this is only because here I chose to draw P-O as a single bond, instead of its resonance form with a P=O double bond.
(Advanced) References and Further Reading
- The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products
Oyo Mitsunobu
Synthesis 1981 (1) 1-28
DOI: 10.1055/s-1981-29317
Classic review on this reaction by Mitsunobu himself. - Preparation of Esters of Phosphoric Acid by the Reaction of Trivalent Phosphorus Compounds with Diethyl Azodicarboxylate in the Presence of Alcohols
Oyo Mitsunobu, Masaaki Yamada, Teruaki Mukaiyama.
Bulletin of the Chemical Society of Japan, Volume 40, Issue 4, April 1967, Pages 935–939
DOI: 10.1246/bcsj.40.935
The original report of the Mitsunobu reaction, used for making phosphorylated alcohols. - A GENERAL PROCEDURE FOR MITSUNOBU INVERSION OF STERICALLY HINDERED ALCOHOLS: INVERSION OF MENTHOL.
Jeffrey A. Dodge, Jeffrey S. Nissen, and Misti Presnell
Organic Syntheses 1996, 73, 110.
DOI: 10.15227/orgsyn.073.0110
This is a procedure from Organic Syntheses, a collection of organic chemistry procedures that have been independently reproduced and are thus extremely reliable. In this example, the hydroxyl group of the alcohol menthol is inverted using a Mitsunobu procedure. - Mechanism of the Mitsunobu Reaction: An Ongoing Mystery
Elizabeth A. Kroll; Oyhun Kwon
Synthesis (Stuttg). 2024 Jan 24;56(12):1843–1850.
DOI:10.1055/a-2232-8633
(From the abstract): The Mitsunobu reaction is one the most widely known reactions in the organic chemistry canon. Despite its fame, some aspects of the mechanism remain poorly understood, 55 years after its initial discovery. This short review collates the findings of several publications focused on the mechanism of the Mitsunobu reaction, highlighting both the current state of knowledge and the remaining missing pieces.