Reduction of Amides to Amines
Description: Amides can be reduced to amines with a strong reducing agent like lithium aluminum hydride (LiAlH4).
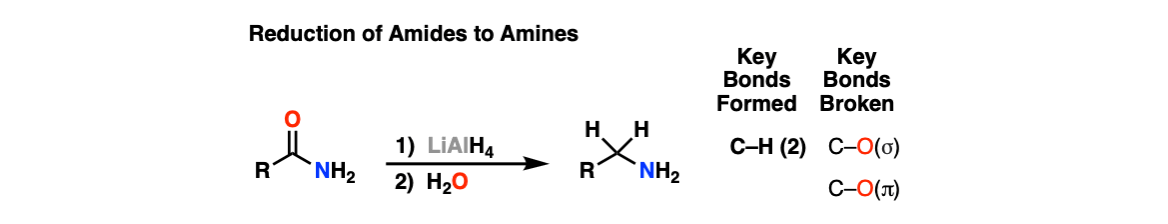
Notes: The purpose of water at the end is for “workup”, which neutralizes strongly basic reagents at the end of the reaction.
Examples:
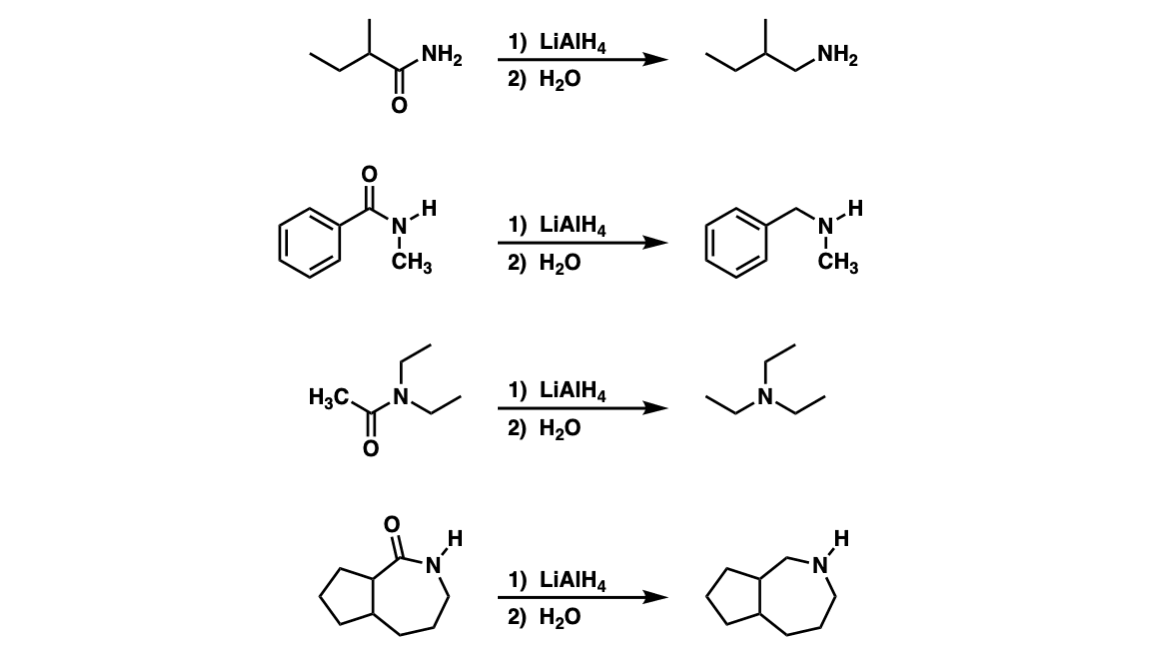
Notes: Note that this reaction can work for primary (Example 1), secondary (Example 2), and tertiary (Example 3) amides. The last example shows a cyclic amide (a “lactam”) which results in a cyclic amine.
Mechanism:
The first step is addition of hydride (from Al-H) to the amide carbonyl (Step 1, arrows A and B) followed by elimination (Step 2, arrows C and D) breaking the C-O bond, and then addition of hydride ion to the iminium ion (Step 3, arrows E and F) to give the amine.
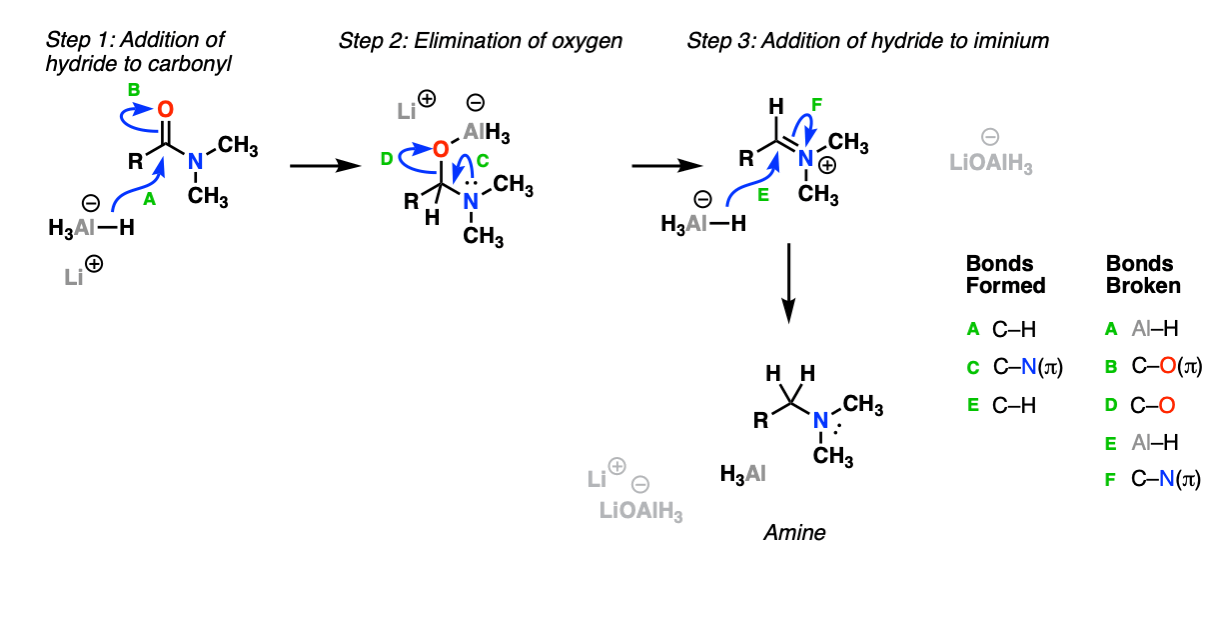
Notes: Amides are considerably more difficult to reduce than esters due to the fact that the nitrogen lone pair donates strongly to the carbonyl carbon.
Real-World Examples:
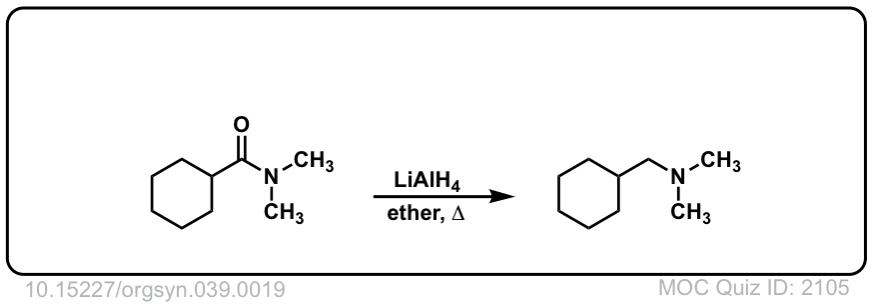
Org. Synth. 1959, 39, 19
DOI Link: 10.15227/orgsyn.037.0019
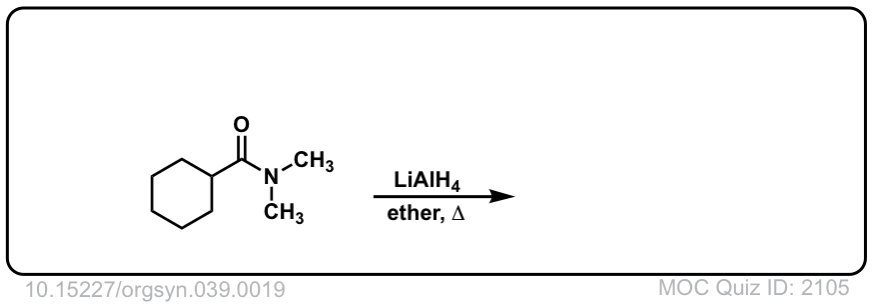 Click to Flip
Click to Flip
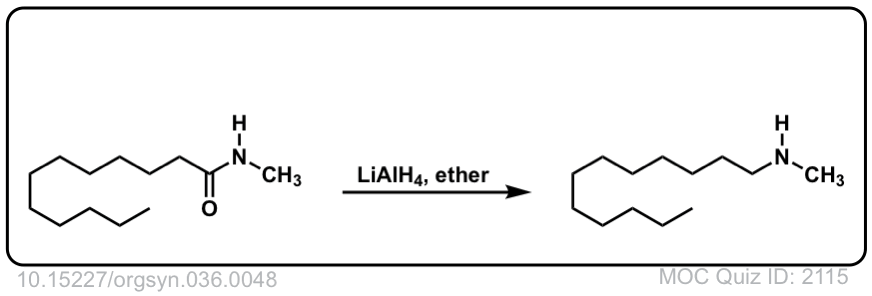
Org. Synth. 1956, 36, 48
DOI Link: 10.15227/orgsyn.036.0048
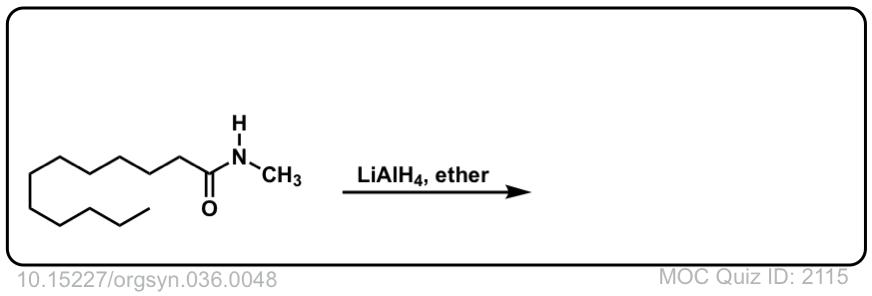 Click to Flip
Click to Flip