Conversion of carboxylic acids to esters using acid and alcohols (Fischer Esterification)
Description: When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). This reaction is called the Fischer esterification.
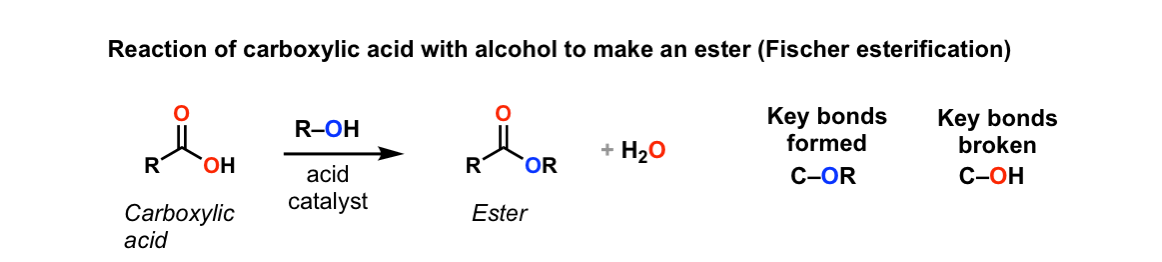
Notes: The reaction is actually an equilibrium. The alcohol is generally used as solvent so is present in large excess. Many different acids can be used; it’s common to see just “H+”, although H2SO4 (sulfuric acid) and TsOH (tosic acid) are also often used.
Examples:
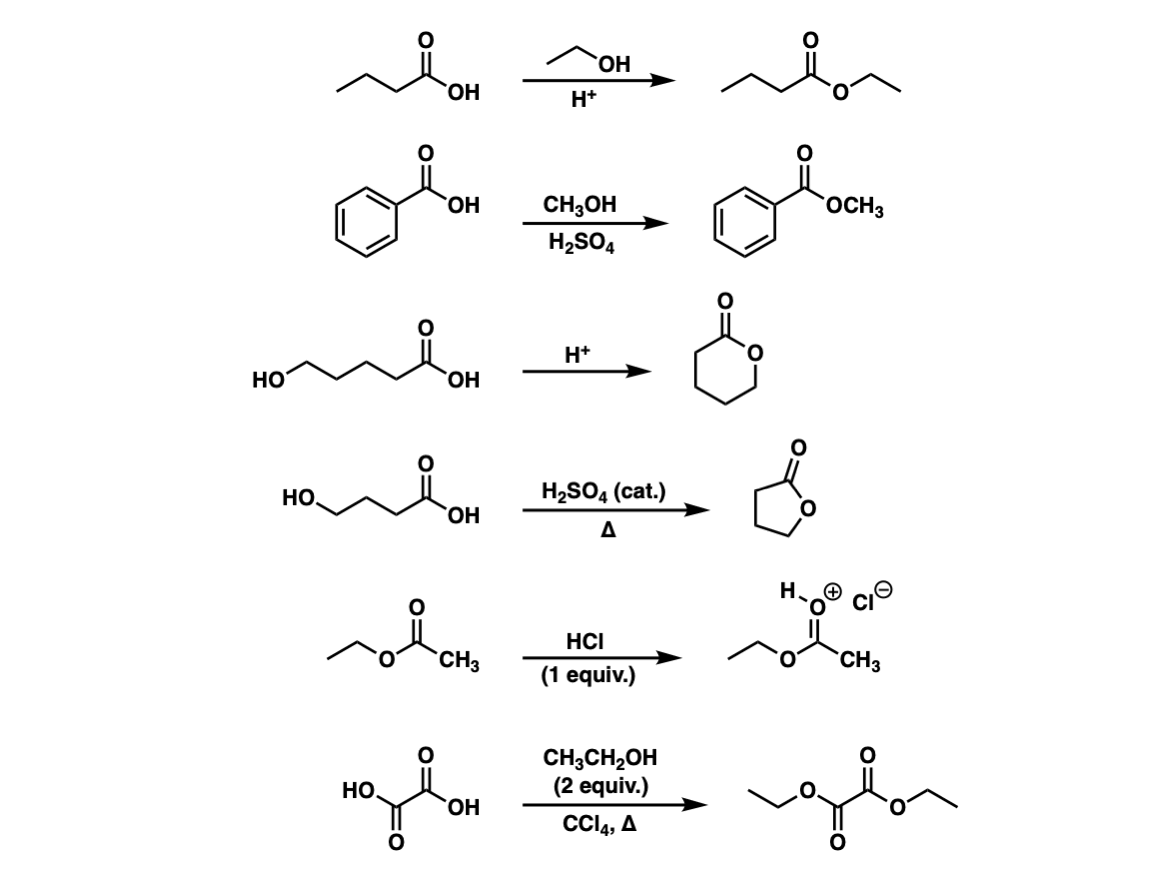
Notes: The byproduct of each of these reactions is water. Note that the third and fourth examples are intramolecular reactions that form a cyclic ester. Cyclic esters are also called lactones.
The fifth example shows that without any added alcohol, the only thing that happens is protonation of the carbonyl!
The sixth example is a double Fischer esterification.
Mechanism:
For such a seemingly simple reaction (replacement of OH by OR) there are actually a lot of steps. Protonation of the carbonyl oxygen by acid (Step 1, arrows A and B) makes the carbonyl carbon a much better electrophile. It undergoes 1,2-addition by the alcohol (Step 2, arrows C and D) whereupon the proton from the alcohol is transferred to one of the OH groups (Step 3, arrows E and F). Subsequent 1,2-elimination of water (Step 4, arrows G and H) leads to the protonated ester, and the ester is then deprotonated (Step 5, arrows I and J).
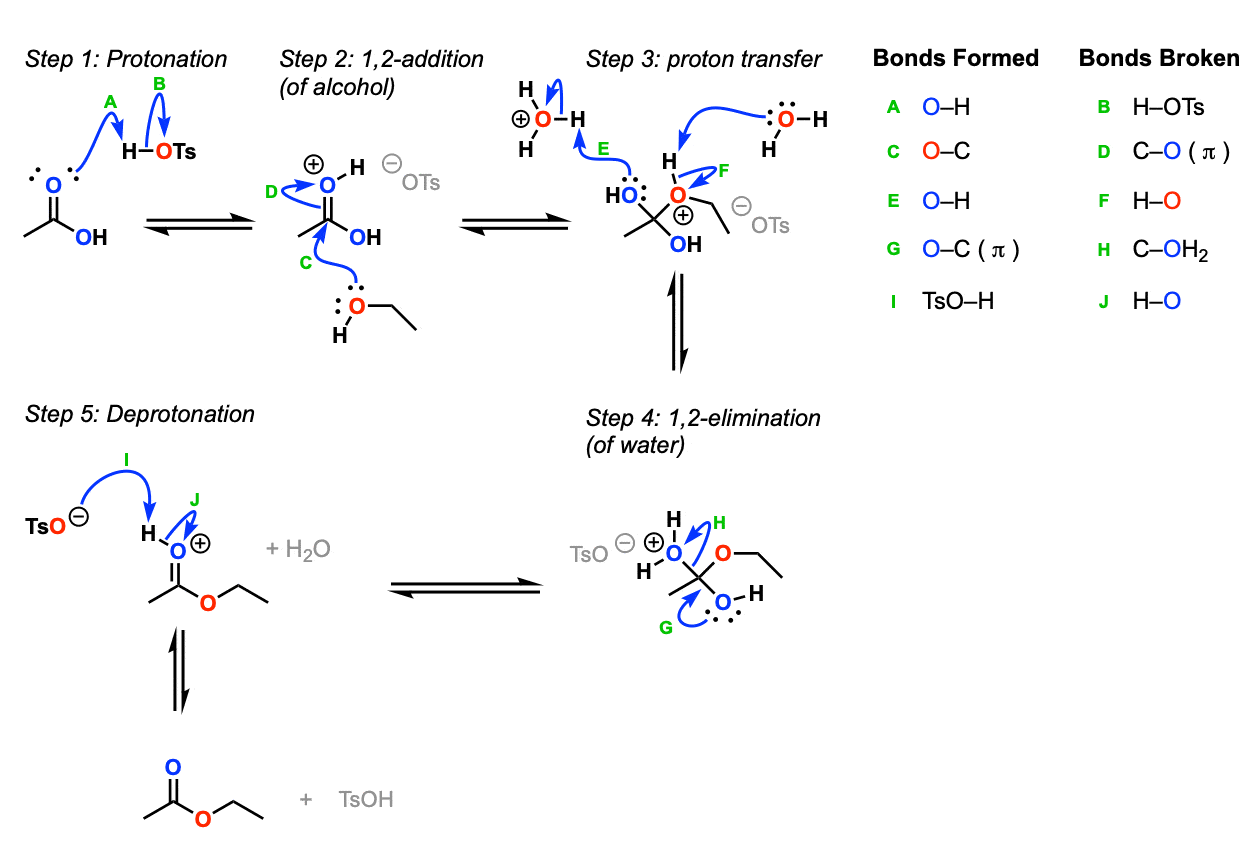
Notes:
- All of these steps are in equilibrium
Note that the acid is a catalyst here (regenerated at the end) and serves two purposes. First, it makes the carbonyl carbon a better electrophile (Setting up step 2) and also allows for the loss of H2O as a leaving group (much better leaving group than HO–)
(Advanced) References and Further Reading
- First example
Emil Fischer, Arthur Speier (1895). “Darstellung der Ester”.
Chemische Berichte. 28: 3252–3258
DOI: 1002/cber.189502803176
Original paper by Emil Fischer and Arthur Speier describing acid-catalyzed esterification of carboxylic acids and alcohols. - Protonic States and the Mechanism of Acid‐Catalysed Esterification
Dr. H. Zimmermann Dr. J. Rudolph
Angewandte Chemie Int. Ed., Volume 4, Issue 1, January 1965, Pages 40-49
DOI: 10.1002/anie.196500401
Considerations of proton mobility in the condensed phase suggest a two-step mechanism of esterification, which proceeds via a tetrahedral intermediate. - Ethyl Adipate
M. Micovic
Org. Synth. Coll. Vol. 2, 264
DOI: 10.15227/orgsyn.000.0001
One of the first procedures in Organic Syntheses, a reliable source for reproducible organic transformations. This uses a Fischer esterification to convert adipic acid, a diacid and precursor to nylon-6,6, to ethyl adipate.
The ester volatility temperature is high. For example PEG400 oleate. In this reaction, for the transport of water, toluene and nitrogen gas and water are collected in the dean stark and returned to the toluene reaction medium with reflux. Toluene at 175 degrees is removed by vacuum. But the toluene smell remains in the ester. Which solvent would be as suitable as toluene to carry water and no odor in this process?
If we use toluene to carry the water from the reaction, which solvent do you think I should use so that there is no toluene odor in the ester formed after vacuum? Should it form an azeotrope like toluene and separate easily after esterification? I will be glad if you answer.
How volatile is your ester? If you have traces of toluene left in your ester after the reaction, you could always take it up in a little bit of n-pentane or diethyl ether to get rid of any traces of toluene.
If we want to use dean starck setup, it means we have to use Toluene as solvent?
then what what would be nucleophile source if I want to make methyl ester?
please reply.
Hello, I use diethyl ether as a solvent to extract chlorinated Herbicides. In order to get rid of the water that could probably get into the sample, I let my sample sit in Acidified Na2So4 before concentration and Esterification. is there anything better I could do (if Dean-Stark trap isn’t an option)? Also, the standard method suggests using a mixture of methanol/2,2,4-Trimethylpentane/ ethyl ether/diazomethane, and silicic acid added respectively but I don’t understand the role of the iso-octane and the silicic acid in the reaction!! should I be adding them or the diazomethane and methanol would be enough?
Thanks in advance
Without seeing your specific example I can’t give you a good answer. From what you’ve written I don’t know the structure. I can tell you that diazomethane is something you absolutely should not work with unless you have been properly trained in its use by an expert. It is potentially very explosive.
Is it Sn1 or Sn2 ….and how to determine nucleophilicity of alchohol ,that is which alcohol will react first.
Side reactions?
Depends on the substrate. A very high yielding reaction with simple starting materials, but if you have acid-sensitive functional groups that aren’t protected, inevitably there will be complications.
i couldn’t understand what’s OTs there?
Tosylate, or p-toluenesulfonate. https://www.masterorganicchemistry.com/2015/03/10/tosylates-and-mesylates/
Hye, im wondering why does esterification process starts at 30°C?
Where did you get that information from?
If my alcohol is solid, what solvent can i use ?
Uh oh. What solid are you trying to use? Phenol?
Why don’t you convert the carboxylic acid to an acid halide and then take it up in an appropriate solvent and add your solid alcohol.
Hi,
I am going to write my extended essay on chemistry, and I came up with a topic which is “what is the effect of different concentrations of sulfuric acid as a catalyst of esterification on the yield of ester when ethanol is reacted with ethanoic acid”, but my teacher said that it is too easy for an extended essay. And I am considering about the other methods that increases the yield of ester and make comparisons with the esterification that uses sulfuric acid. So any suggestions to me to make my topic more complicated?
Thank you so much
I don’t have a good answer for you. One thing you could look at is the effect of various substituents on the para position of benzoic acid and how the affect the rate of the Fischer esterification with an alcohol like ethanol. This is referred to as a Hammett plot and it gives useful information about the mechanism.
Hi,
I am synthesizing dimethyl malate with dl-Malic acid and excess methanol with sulphuric acid as the acid catalyst. Using HPLC, I can identify 3 peaks (depending on my reaction condition). Using a standard as a check, the major peak is the dimethyl ester and another is definitely a dimethyl fumarate. The other peak based on GC is speculatively dimethyl succinate. The standard almost agreed with this but my mole balance based on yield% for each does not add up and the so-called succinate seems to be the problem. Truth is, I do not know how a succinate could be produced following traditional reaction mechanisms for fischer esterification reactions. Based on the wealth of your experience, what do you think the other side product is? I have scoured the internet and journals for ideas and everything seems to be a blur.
Probably not dimethyl succinate. You could do a michael addition of methanol to dimethyl fumarate and you’d get a very similar molecular mass to dimethyl succinate.
Hey!
I’m about to venture into synthesizing different esters for perfume and lotion and soap production.
What should I ensure?
Thanks
If you have to ask, I’d suggest you buy them instead of making them.
Hello, I need to make a esterification using allylic alcohol that is very reactive with sulfuric acid, there is any other substances like acid resine that I could use. Thank you very much.
Why don’t you just convert the carboxylic acid to the acid chloride and do it under basic conditions?
Hi. How do I compare the rates of acid catalysed esterification for different carboxylic acid reagents? What role do electronic effects play in determining the reactivity of a carboxylic acid towards Fischer esterification?
One way you could do it is by looking at the linear free energy relationship (LFER) for the Fischer esterification reaction and then getting the Hammett parameters for the substituents. That would provide you with your answer. It would require some digging through the primary literature.
Is Fischer esterification an SN1 reaction or an SN2 reaction?
Please explain…
It is neither – it is an acyl substitution reaction!
Why basic medium is not taken instead acidic medium…
It’s possible to use basic conditions but that would be “transesterification”. The Fischer employs acid.
What is the difference in using a Dowex-resin and sulfuric acid as a catalyst? Would the isolation of the product need to be different?
Well the nice part about using the resin is that you just filter it off, instead of having to neutralize the solution during workup.
If i wanted to turn a fully organic Carboxylic acid into its corresponding methyl-ester, which is regarding safety and environment the better method: 1) Fisher esterification or 2) Alkylation with Methyl iodide in basic circumstances? And because of which safety and environmental reasons exactly?
Well, you probably want to use the Fischer. Methyl iodide can be effective but it isn’t a lot of fun to work with, it’s a mutagen and will alkylate your DNA if you get a few drops on your hand.
Why is there removal of -OH from carboxylic acid but not from alcohol to give water?
In order to have water as a leaving group you have to form two O-H bonds. That’s not possible with the alcohol because you’d have to break an O-C bond, which would be much more difficult.
Hi, two questions,
when the water is eliminated, is there any preference for it being oxygen from the carbonyl or from the hydroxyl of the carboxyl group?
and is there evidence for the proton transfer involving an external (e.g. alcohol) molecule rather than an internal transfer?
Thanks!
No, in experiments with isotopically labelled oxygen scrambling of the isotopic label is observed, meaning that there is no preference. As far as proton transfer, it’s probably an external molecule but that’s a tough question to answer.
Why can’t the Fischer esterification take place under basic catalysis?
Because your leaving group would be O (2-) which is a terrible leaving group.
Why doesn’t salicylic acid undergo intramolecular esterification?
It’s way too strained. Look at a model. 4 membered ring, it would break apart too easily.
Why is it necessary to add sulphuric acid in the synthesis of esters?
Otherwise your leaving group would be HO- , which would be a very slow reaction.
I am doing esterification with ethanol getting conversion 95 to 96 %area purity by HPLC. but i am getting yield 81 to 82 %.
please suggest work-up conditions of esterification.
It’s not uncommon to see a drop in yield comparing HPLC yields to isolated yields. Workup is just a standard wash with saturated bicarb followed by 3x extraction with appropriate solvent. Search the literature, you’ll find hundreds of examples.
Is esterification a nucleophilic substitution reaction or electrophilic substitution reaction?
Nucleophilic acyl substitution.
Why benzoyl chloride is used instead of carboxylic acid with phenol in synthesis of phenyl benzoate
Phenol is a pretty good leaving group owing to the fact that the phenoxide ion is a weak base, therefore the Fischer doesn’t work as well. I’d prefer to use benzoyl chloride too.
Why does the carboxylic acid need to be in excess over the alcohol in this reaction? It is something I found out today in lab and do not understand why.
I think you mean alcohol in excess over carboxylic acid. This is done because the byproduct of the Fischer is H2O and the reaction is in equilibrium with the backwards reaction (aqueous hydrolysis of ester). By using a large excess of alcohol, the ratio of the concentration of alcohol to water will be high, and this will result in a higher equilibrium constant for the forward reaction.
How come the oxonium ion (protonated alcohol) is not generated and rather the carbonyl carbon is protonated by acid. I thought that the carbonyl carbon is more stabilized as neutral and that the oxonium ion gets deprotonated by the carbonyl oxygen of the carboxylic acid. Then alcohol attacks carbonyl carbon that’s dbl bonded to a protonated oxygen kicking electrons up making oxygen neutral and forming tetrahedral intermediate with two OH groups. Base comes in and deprotonates ester H. One OH group deprotonates BH+, other OH Group sends its electrons down forming dbl bond and kicking off water as a leaving group. Then Base comes in and deprotonates last H on newest dbl bonded O forming carbonyl group of ester. That was the mech I was taught in class
It could be. But the carbonyl oxygen is more nucleophilic.
I made an esterification of an amino acid with a long chain alcohol in excess, the reaction occurred in the carboxyl group, but my ester was not soluble in water, as I can do to make it soluble, and my only alternative would be to tinker with the amino group.
This is normal for the Fischer. Your product will be quite soluble in organic solvents. You might find the water solubility of the resulting product changes greatly with pH.
The reason could possibly be visualized by the mechanism as well; the protonated hydroxyl group sets the stage for the hydroxyl group to leave; this is of course facilitated by the oxygen of the hydroxyl group on the alcohol making an attack` on the carbonyl group of the carboxylic acid. Thus the hydroxyl group of the carboxylic acid is destined to leave and the oxygen of the alcohol moiety stays with its hydrogen destined to leave. Therefore we see that the OH of the carboxylic acid and the hydrogen of the alcohol leave to make the ester. See the illustrated mechanism of esterification above…
How can I get more than 95 % of ester by reaction between carboxylic acid and alcohol? Excess acid or excess alcohol needed?
Excess alcohol, and it’s best to sequester the water somehow. A Dean-Stark trap is a good way to do it.
Suppose I were producing ethyl nicotinate from nicotinic acid and ethanol with H2SO4, is it possible that the N on pyridine gets protonated by the acid?? Is it a cause of concern?How can I prevent that?
Yes, it is absolutely a concern, because now the strongest acid in solution is protonated nicotinate. You might consider changing the concentration of something… : – )
Hi, I was wondering why the alcohol must be excess rather than the other reactant to drive the reaction forward.
Usually the alcohol is less precious than the carboxylic acid, and it can serve as the solvent (ethanol for example).
The third step (proton transfer) can’t be an intramolecular reaction; doing so would form a 4-membered transition state, which is very high-energy and disfavored.
Granted. I should probably change this. Thanks for your comment.
I have conducted esterification by using lauric acid and methanol over solid acid catalyst. The product was analyzed by using GC and I found out that at particular catalyst (let say ZS) GC only reveals one peak which when I compare it with standard it is methyl lauric and no width peaks of lauric acids. So i called it conversion 100%. But when I calculated using (%peak area x w product)/w sample, I obtained my yield FAME less then 100% (max 89%)
How could this happen, whereas my GC result only showed one peak.
Thank you
What are other safer alternatives as a catalyst instead of H2SO4?
p-toluenesulfonic acid (p-TsOH). Also, a particularly mild acid is PPTS (Pyridinium para-toluenesulfonate).
Hi, I’m working with graphene oxide (COOH), and going to do PEGylation (OH), is it possible to just add this tow reagent for this modification, or need to have any other kind of acid as a catalyst?!!!
I would do an extensive literature search for this reaction and not try to re-invent the wheel. I can’t imagine someone hasn’t tried esterification of graphene oxide already.
In a Fischer esterification reaction, you can remove excess carboxylic acid from the reaction mixture by adding Na2CO3. But what would be a side reaction if the solution was strongly basic with aqeuous sodium hydroxide?
Your reaction product is an ester. What reactions can esters undergo with strongly basic aqueous sodium hydroxide?
why we can’t use the con. H2SO4 during esterification?
Do you really want to boil concentrated H2SO4 when you don’t have to?
If I lable The oxygen of the alcohol as O-18.. I should find that Oxygen in the Ester.. But back here I found in a book that it holds true with primary alcohols but with tertiary alcohols … O-18 is not there… What’s the reason for that?
Think about what happens when you heat a tertiary alcohol with acid.
What if we are using Isonicotinic acid with catechol, should the simple esterification work using catechol in excess with H2SO4 as catalyst. Or should we prefer to go through acid chloride formation followed by esterification?
Whatever the answer please give reason!
You might ask if isonicotinic acid has any basic atoms that might react with catalytic H2SO4.
Hello
When would it be impractical to use an excess of alcohol to drive the equilibrium toward products?
Usually when your alcohol is precious. Then I’d go through the acid chloride instead. Another situation is phenol, which is a solid and a good leaving group besides.
What happens if Acid is in excess and which is better catalyst PTSA or Sulphuric acid? And why one is better than other? What if other one is used?
They’re equally strong acids, just a matter of convenience really. Another option is anhydrous HCl, which can be made by adding acetyl chloride to the alcohol.
hii,
can heating be used as a catalyst instead?
No, acid is required.
Why does the protonation of the carbonyl oxygen by acid make the carbonyl carbon a better electrophile? Why it becomes a better
electrophile?
When the carbonyl oxygen is protonated, the resonance form where there is a positive charge on carbon will make a greater contribution to the resonance hybrid. Another way of looking at it is that it weakens the C-O pi bond.
When butyl hexanoate is heated with a large excess of methanol in this reaction, will methyl hexanoate be the major product? (Because its hard to believe that MeO- replaces BuO-)
If you use methanol as solvent, you should get transesterification under these conditions. But why not just treat butyl hexanoate with a large excess of sodium methoxide?
for cyclohexylbenzoate who is best
ficher esterification or acid cholaride with alcohol and why?
What ester are you trying to make, and on what scale?
Can Carboxylic acids and thiols undergo reaction like esterification??
No, the best route is to go through an acid chloride and then add a thiol. Nobody wants to use pure thiol as solvent… yuck!
Why does ester has sweet fruity smell
Evolution. Can’t be more specific than that.
Hi ,I have a question though, where in our exercise we synthesized isoamyl acetate
Why is acid catalyzed hydrolysis not favored when upon extraction, where NaHCO3 was used as the extracting solvent for excess acetic acid where the bicabonate ions form water and carbon dioxide, wouldn’t it trigger additional water concentration and shoft the reaction equilibrium to the left, favoring the formation of reactants?
Most esters are stable enough to survive aqueous extraction under basic conditions.
What possible side products would be formed in this reaction?
Thanks in advance
Hi Amanda – for a simple carboxylic acid and a simple alcohol, it’s generally a very high-yielding reaction (>90% yield). It’s possible to form some byproducts of dehydration, such as loss of water from the alcohol to give an alkene or combination of two carboxylic acids to give an anhydride. However these should be fairly minimal. Again, though, it depends on the structure of the starting materials. You would want to avoid having any acid-sensitive functional groups on either molecule because they may lead to side reactions.
Hi, I need to get dry product, Tell me how to remove water at the end of the reaction?
2nd question, After getting dry product i want to react the product with hydrazine hydrate? I need catalyst or not? if yes, then which one?
Thanks in advance
Dean stark trap. That does the best job.
What is the rate determining step in this reaction ?
I don’t have the literature with me to back it up at the moment, but generally, the rate determining step is attack at the C=O
Thanks, and great work.
Can you suggest three ways in order to shift the equilibrium in favor of the ester?
Remove water. A Dean Stark trap is the usual trick.
I did esterification of palmitic acid by a tertiary alcohol using an acid activated resin as catalyst. why conversion using tertiary alcohols failed to yield esters. Thank you
Hard to say. Are you following literature precedent?
in acidic media tertiary alcohols dehydrates and no longer remains as alcohols
Yes.
Hey James,
I have a question. How can you get rid of excess ethanol in this procedure?
I’m assuming after you’ve made the ethyl ester. Rotary evaporation.
How to form a molecule with two ester groups?
If you heat up a carboxylic acid with acid, you may form an anhydride. Works best for simple systems. Is that the question you were asking?
Can we use hydrochloric acid for the reaction with a Dean-Stark trap to remove water formed. Would the water in hydrochloric also distil over.
HCl can be used. As for volatility, the HCl will react with solvent or water forming a conjugate acid (e.g. H3O+ Cl-) . This is a charged species and is not volatile, therefore it will not boil over.
HCl is slightly less preferred because sometimes at elevated temperature the Cl- counterion can act as a nucleophile, leading to undesired substitution byproducts. This is not a problem with the HSO4- ion.
If there any possible carboxylic acid can react with secondary alcohol
Yes, isopropanol for example. The major restriction is the fact that the alcohol must be used as solvent.
Hi,
Why does it need be used as solvent? Is it because to make sure that we have huge excess of alcohol?
Will it work if the secondary alcohol is not in solvent form? (Its in solid form and Im thinking of using THF as a solvent)
If you don’t want to have a huge excess of alcohol, then I suggest converting the carboxylic acid into an acid chloride and forming an ester using Schotten-Baumann conditions.
Hey James,
Suppose I wanted to create an ester linkage by grafting a aromatic carboxylic acid on to a long chain polyol with a medium density of hydroxyl groups. Would using an acid catalyst work and give me a high yield? or would I need to use a different type of esterfication rxn/catalyst? right now I am using nmp as a solvent
Any suggestions?
hi, can i dilute the acetic acid directly in the ethanol? and what is the amount of ethanol and acetic acid (in L or molarity) be in order to make the esterification irreversible? thanks.
One drop of concentrated H2SO4 usually does the trick.
Why hydrogen is removed from alcohol and hydroxyl group from carboxyl group during esterification why not reverse of it? And how can we determine this fact?
Choice of solvent. To get the ester, use the alcohol as solvent. To do the reverse, use water as solvent.
In Step 1, the proton attacked the O of the C=O of the COOH. Why didn’t it attack the O of the OH of either the alcohol or the COOH?
It could, but the oxygen of the carbonyl is more nucleophilic. If you draw the resonance form of the carboxylic acid you’ll see that a lone pair from the OH can donate into the carbonyl , breaking the C=O pi bond and resulting in a negative charge on oxygen. That’s the best nucleophile.
How does the catalyst generally work in this reaction? What reacts with the sulfuric acid and does it (H2SO4) need to be concentrated or diluted and why?
Thank you :-)
Dilute H2SO4 or TsOH is fine. It’s catalytic.
Ok, what could reasons be for a hydrolysis reaction that is expected to be a first order reactions turns a zero order reaction ??
It is reversible reaction, alcohol ( one of the reactants) must be in excess based on lechatliers principles
Yes, exactly Balakrishna.
Why is the ester hydrolysis not observed in this reaction? (acid is in excess)
Ester hydrolysis is not observed because the ALCOHOL is in excess of water (we use the alcohol as solvent). To get the reaction to run the other direction we use water as solvent.
thank you :)
Hi,
I was wondering if the reaction could still take place if the CO2H was attached to a benzene ring with a Nitro group in the para position? Would this react with a Phenol?
Thanks in advance
Yep, you’d make an ester.
Hi, do you by any chance know some examples of research where Fischer esterification is used? What are its real life applications?
Thank you.
See the references section.
What is the effect of adding aq. HCl on the equillibrium ?
If you add aqueous acid it will drive the reaction toward the carboxylic acid.
What is the purpose of water during this step. In the condensation flask?
Water is formed during this reaction as a byproduct.
in certain reaction we use thionyl chloride instead of acid catalyst,why??
In those cases you are converting a carboxylic acid to an acid chloride. It’s not the Fischer esterification.
That’s not entirely correct – thionyl chloride/alcohol is also an effective way to carry out esterification (see Tetrahedron Letters, Vol. 37, No, 35, pp. 6375-6378). Thionyl chloride reacts with alcohol to generate the requisite acid catalyst in situ.
Yes – thank you for mentioning this method for adding anhydrous HCl to the reaction mixture.
Would this reaction work using a base as a catalyst?
No, it would not. Base just forms the conjugate base (carboxylate) and the reaction stops there.
How about in the case of mevalonate forming a cyclic ester to become mevalolactone? Mevalonate is in its carboxylate form…I am trying to understand the proton transfers that occur in this cyclization reaction/equilibrium under physiological conditions.
1. Why is the carboxylic acid and alcohol not reacted in equimolar amounts
2. Why is it necessary that the aqueous solution is basic?
1. The reaction is an equilibrium. Using an excess of alcohol drives it to the right (ester) side.
2. The aqueous solution is ACIDIC in order to 1) speed up addition of the nucleophile to the C=O group, and 2) convert OH into OH2+, making it a better leaving group.
what happens if dicarboxylic acid reacts with alcohal?
Depends on the structure of the dicarboxylic acid. Could get a diester. Might not.
there is any case other than acid hydrolysis of esters??
Sure, esters can be hydrolyzed with base.
sir ,, what is the case in which acid is not used in process of ester formation?? thank you
Using diazomethane (CH2N2) is one common example. Great for methyl esters. Another is treatment of acyl halides with NaOR.
sir why does the protonation not occur at the hydroxyl group of alcohol?
It does, to some extent, but that reaction is reversible and it doesn’t help the reaction progress forward.
Double bonded oxygen has more nucleophilicity for protonation than hydroxyl group oxygen I think so…
Yes, you are correct.
how the reaction between alcohol and TsOH is reversible and the differenne between Pka value is 19 unit (approxomatly)
Hi Harith – the number you need to be looking at is not the pKa of the alcohol (which measures the equilibrium for formation of its conjugate base ROH –> RO- + H+ ) but instead protonation of the alcohol to give its conjugate acid (ROH + H+ —> ROH2+ ). The pKa of that species is about -2 , which is roughly equivalent to that of tosic acid. Hence TsOH is in equilibrium with protonated alcohol, as well as protonated carbonyl and protonated water. Hope this helps – James
Ok, thank so much..now it’s clear for me
Hi, sorry…This is not related to ester.
Can you suggest me a test to distinguish between chlorobenzene and benzyl chloride?
Thanks :)
Yeah, benzyl chloride is a hellish lachyrmator and will make you cry. Not the case with chlorobenzene.
why double bonded oxygen donates electron to hydrogen why not oxygen in -OH donates and form +OH2 .
It will happen. But the carbonyl oxygen is more nucleophilic. Check out the resonance form.
Can fischer esterification happen in aqueous solution? In other words, can the protonation in step 1 and deprotonation in step 5 happen via hydrogen ions and hydroxide ions of water, respectively?
Thanks
Hey Ted – the Fischer esterification is in equilibrium with the reverse reaction (acidic hydrolysis of esters to give carboxylic acids).
In order to get the reaction to proceed from acid —> ester, you need to add a large excess of alcohol so that the concentration of alcohol will be much greater than water . This is what drives the reaction toward the ester [think Le Chatelier]
So if you use water as the solvent, nothing will happen to the carboxylic acid. It will just sit there. You need to use an alcohol solvent.
For example if you want to make a methyl ester, add methanol as solvent.
Hope this helps – James
Minor typo: it’s the fourth example reaction that’s an intramolecular reaction, not the third. Great job, as always!
Why is H2SO4 the preferred acid to use as a catalyst over other acids?
This page will show you the reaction including the H2SO4 showing you why it is used as a catalyst there are probably many more catalysts that work but sulphuric acid is mass produced and very cheao
This page will show you the reaction including the H2SO4 showing you why it is used as a catalyst there are probably many more catalysts that work but sulphuric acid is mass produced and very cheap.
TsOH can also be used among many other acids.
Sulphuric acid is also a very good dehydrating agent..So the water produced during the reaction gets absorbed and hence the reaction equilibrium gets shifted in forward direction….
How many mole of acid catalyst that we need to use in this esterification, and is the amount of acid used will effect the reaction?
For practical purposes about 5-10 mol % of acid is common.
how many wt % needed to add the acid catalyst to the reaction ?
A typical value is 10 mol% or so.
why protonation in the first step occures at the carbonyl oxygen not at the hydroxyl oxygen?
carbonyl oxygen has strong Lewis Basic character, with that Lewis acid like H+ reacts fastly.
It is a better Lewis base, yes.
Due to sp2 hyridised oxygen which more reactive then OH group oxygen.
Its due to resonance stabilization of the “Carbonyl-oxygen protonated” intermediate and hence proceeds further unlike that in case of protonation of hydroxyl oygen.
The carboxyl oxygen is more nucleophilic.
Resonance? the carboxyl oxygen has a resonance that puts negative charge on it so it easily picks up a positive hydrogen.
What confuses me though, is carboxylic acid is a much less stable acid than an alcohol and so it seems counterintuitive that it would soak up the extra acid in solution.
Yes, exactly Isaiah.
Would we use methanol for a methyl ester or diazomethane?
Hi Collin –
Diazomethane is great on small scale, and if you want to make the methyl ester. Probably one of my favorite reactions to run because it’s so darn easy.
However the higher analogs of diazomethane (e.g. diazoethane, diazopropane) are not stable enough for routine use. So it’s limited to methyl esters for the most part.
The Fischer esterification is one of those robust, old school, tried and true reactions that can be used to make a huge variety of esters.
Hope that answers your question! James
Hello,
I actually have a question, what would happen if excess watr were added to the ester product and the solution was refluxed under acidic conditions?
I believe that the rxn would occur in the reverse because the rxn is an equilibrium rxn and follows le chatalier’s principle.
Thank you in advance!
Yes, that’s correct. That’s acidic hydrolysis of an ester.
I know if you were to remove the water from the product as it forms, it would drive the reaction towards ester formation, but would the addition of ether to the reactant side of the equation have any effect???
Ether? No.
I have this problem too. I got excess water as my side result. How to remove the excess of water? Because it will decrease the %yield of ester. I added the drying agent, such us sodium sulphate and magnesium sulphate, but it was not doing well. Thank you.
You should search Youtube for videos of a Dean Stark trap in action. It’s very elegant.
I came in expecting an expulsion of the OH similar to the Grignard addition to an Ester – the Electrons rise up the Carbonyl to make C-O(-), then slam back down to make the carbonyl again, and then the O-R leaves because the C-O bond breaks.
Why isn’t this the case here? Is it due to the acidic conditions? Is there a basic version of this reaction that would, in fact, do this addition-elimination reaction?
It’s because -OR is a bad leaving group. If to say that it was -OCl or -OTs, then your thought process would most likely be valid. As is, it would be hard to generate an alkoxide (-OR) in acidic conditions too. I am not sure this reaction would work in base (well not with these starting materials) as it wont go very far from a simple acid-base reaction at the first step.