SN2 reaction of alkoxide ions with alkyl halides to give ethers (Williamson synthesis)
Description: Alkyl halides (or tosylates) will react with alkoxy ions to form ethers. This reaction is called the Williamson ether synthesis.
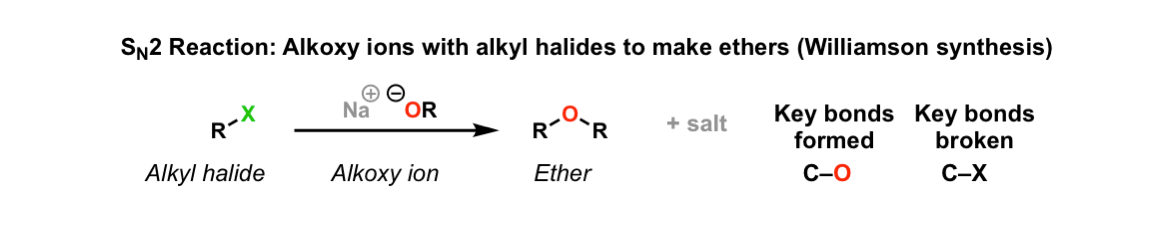
Notes: X here is a halide (Cl, Br, I) or sulfonate (OTs, OMs). The counter-ion on the alkoxy ion can be any alkali metal (e.g. Li, Na, K)
Examples:
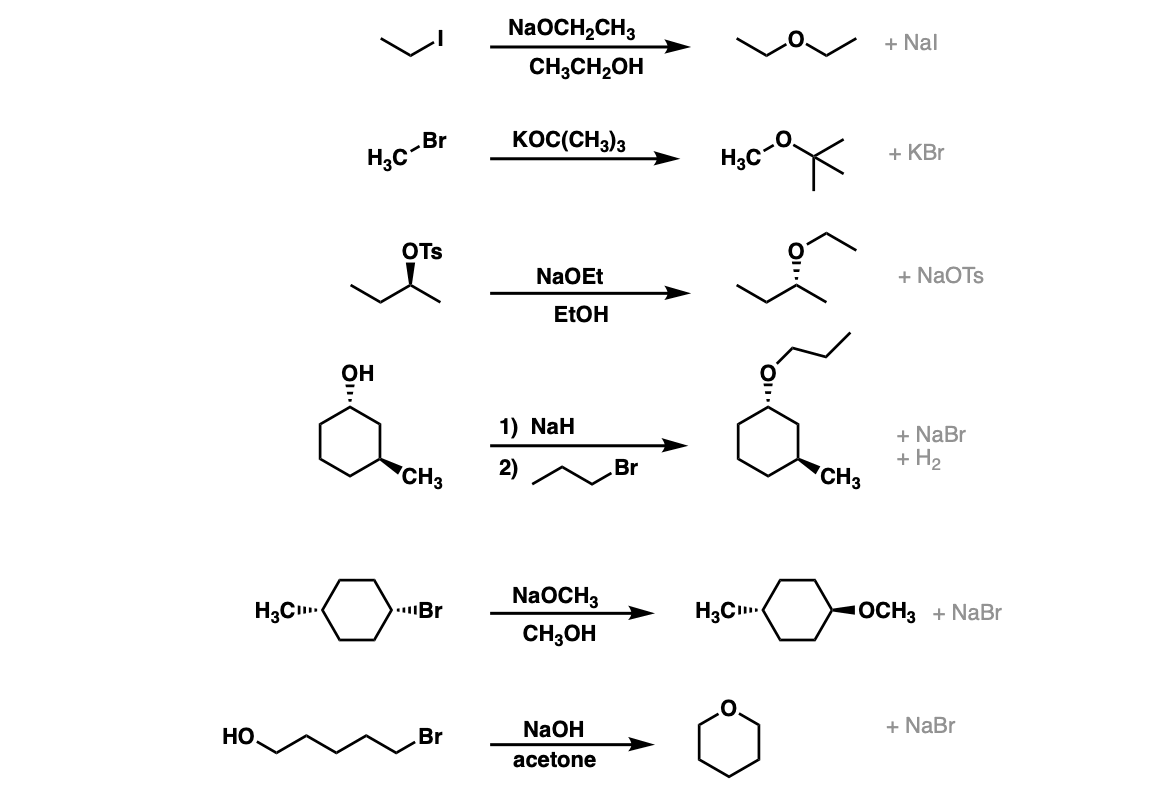
Notes: Note that since this is an SN2 reaction and proceeds via backside attack, there will be inversion of configuration at the carbon (note the last two examples). The best choice of solvent is usually the conjugate acid of the alkoxide.
Note that in the second example that this ether would be difficult to make the opposite way (CH3O- attacking a tertiary alkyl bromide) since SN2 reactions don’t work on tertiary centers.
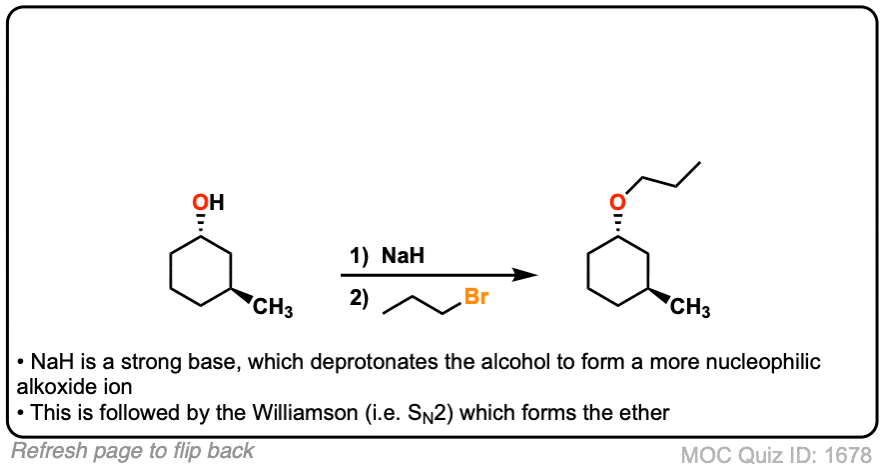
Example 4 shows a deprotonation with NaH to give the alkoxide followed by addition of the alkyl halide. The sixth example is an intramolecular Williamson ether synthesis! Watch out for these types of examples as they are common exam questions.
Mechanism: In the SN2 reaction the nucleophile (RO-) attacks the carbon with the good leaving group, forming a C–O bond and breaking the C–Br bond (Step 1, arrows A and B).
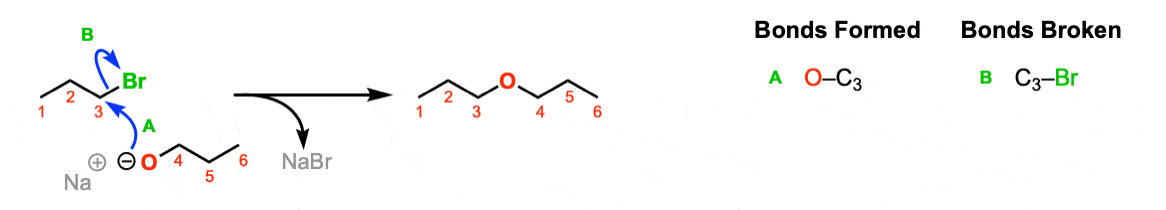
Notes: Again, the Na(+) is not crucial here, it’s just a spectator ion.
Test Yourself!
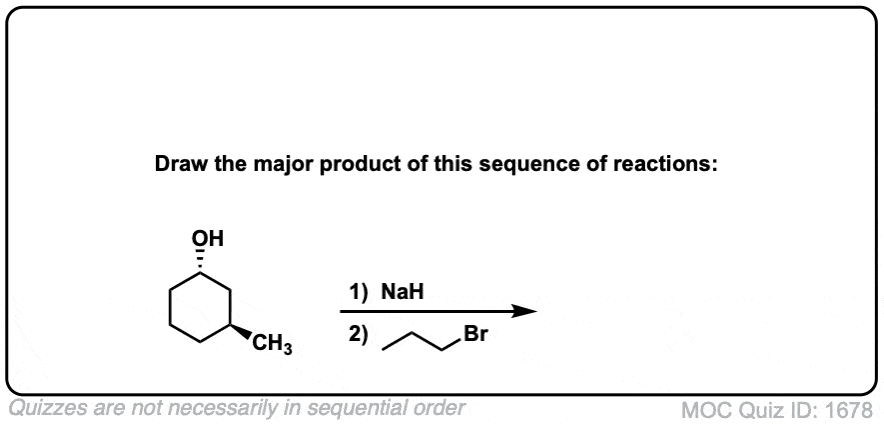 Click to Flip
Click to Flip
(Advanced) References And Further Reading:
- XLV. Theory of ætherification.
Alexander Williamson (1850) , The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 37:251, 350-356,
DOI:1080/14786445008646627
The original Williamson paper. - Equilenin 3-Benzyl Ether
M. Hoehn, Clifford R. Dorn, and Bernard A. Nelson
The Journal of Organic Chemistry 1965 30 (1), 316-316
DOI: 10.1021/jo01012a520
One of the reactions in this paper is a classic Williamson reaction – protection of the alcohol in dehydroestrone as a benzyl ether, using benzyl chloride. - Total Synthesis of (+)-7-Deoxypancratistatin: A Radical Cyclization Approach
Gary E. Keck, Stanton F. McHardy, and Jerry A. Murry
Journal of the American Chemical Society 1995 117 (27), 7289-7290
DOI: 1021/ja00132a047
In modern organic synthesis, the Williamson reaction is used for the protection of reactive alcohols in a substrate. Common protecting groups include methoxymethyl (MOM) and 2-methoxyethoxymethyl (MEM). MOM protection is employed in this total synthesis by Prof. Keck and coworkers.
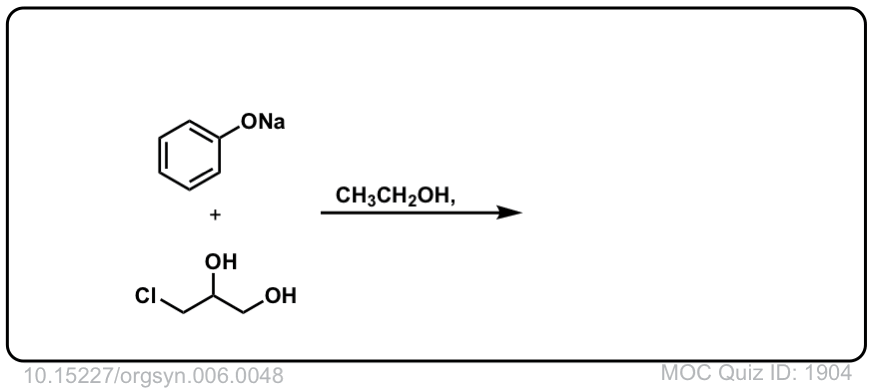
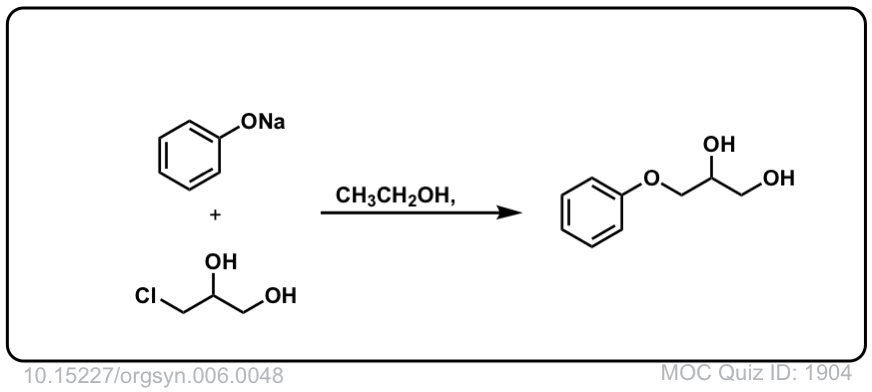
Real-World Examples:
Org. Synth. 1926, 6, 48
DOI Link:10.15227/orgsyn.006.0048
 Click to Flip
Click to Flip
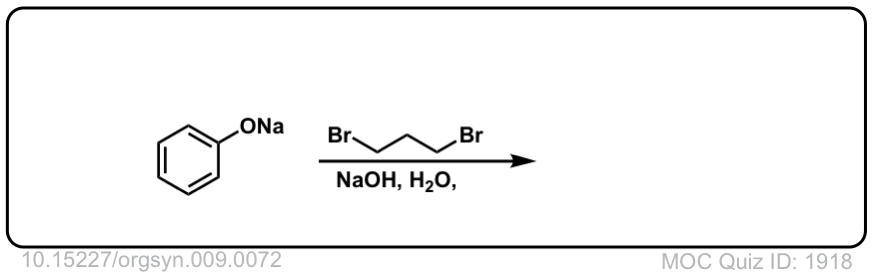
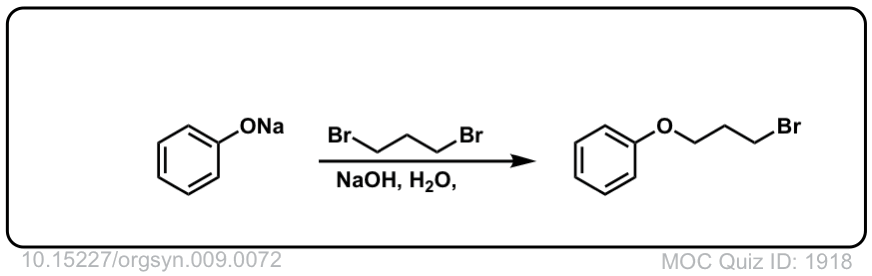
Org. Synth. 1929, 9, 72
DOI Link:10.15227/orgsyn.009.0072
 Click to Flip
Click to Flip
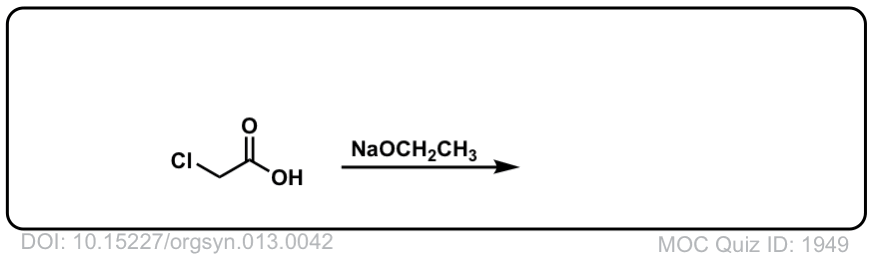
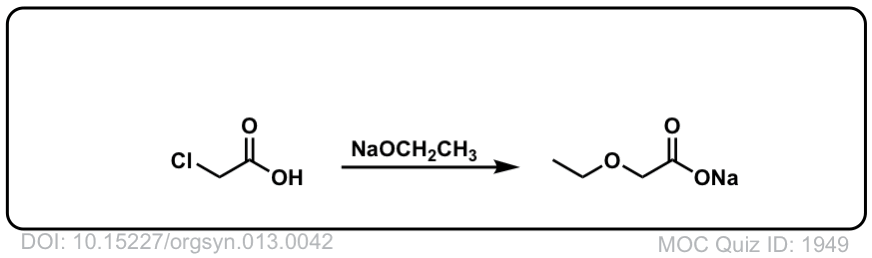
Org. Synth. 1933, 13, 42
DOI Link: 10.15227/orgsyn.013.0042
 Click to Flip
Click to Flip
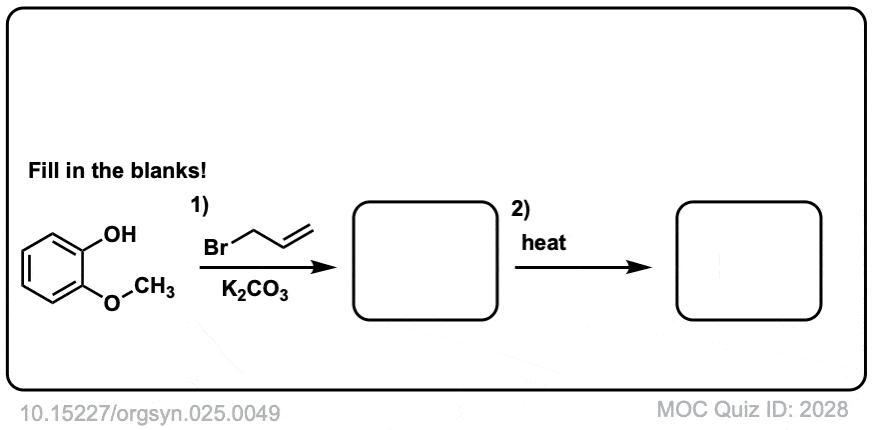
Org. Synth. 1945, 25, 49
DOI Link: 10.15227/orgsyn.025.0049
 Click to Flip
Click to Flip
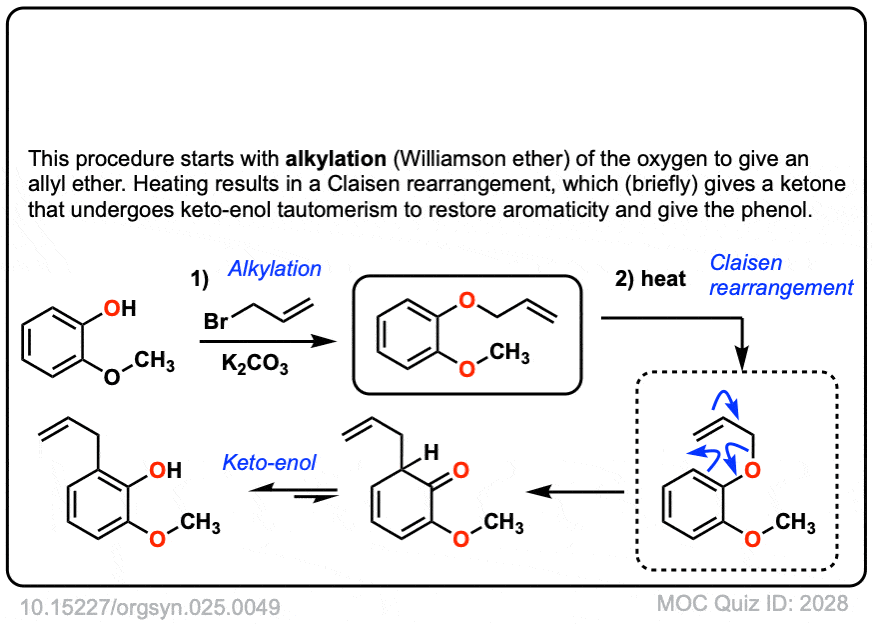
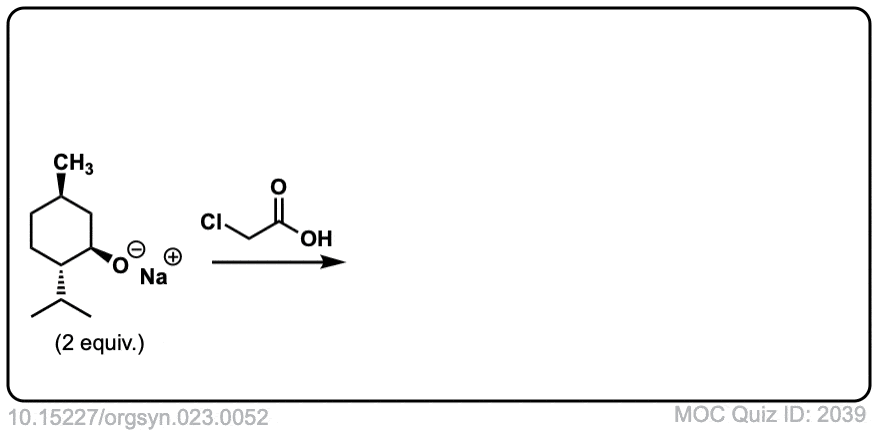
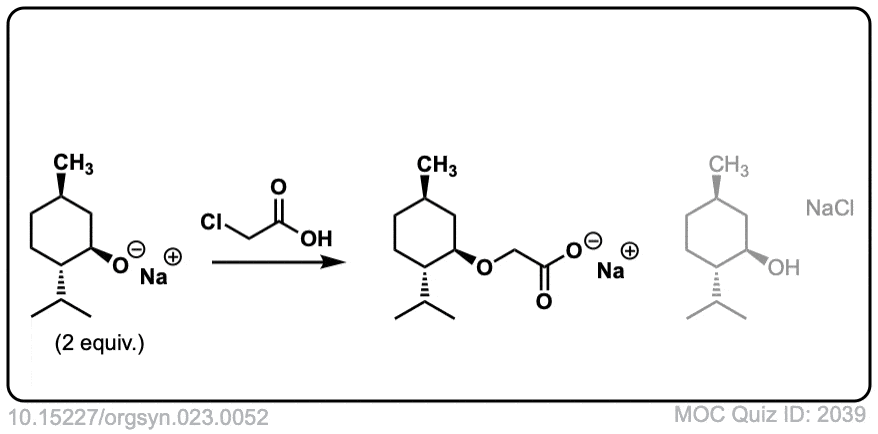
Org. Synth. 1943, 23, 52
DOI Link: 10.15227/orgsyn.023.0052
 Click to Flip
Click to Flip
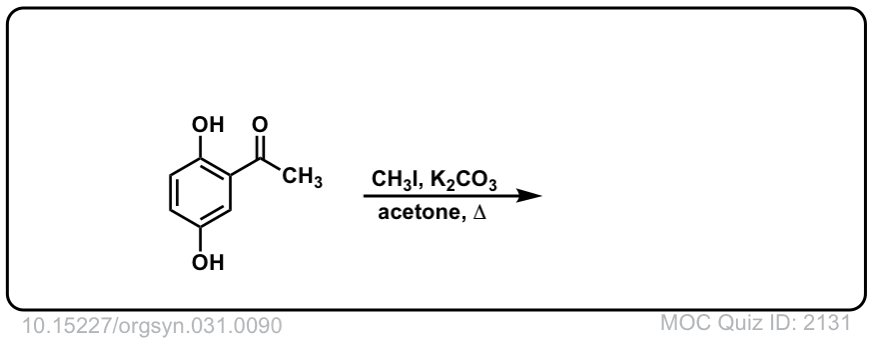
Org. Synth. 1951, 31, 90
DOI Link: 10.15227/orgsyn.031.0090
 Click to Flip
Click to Flip
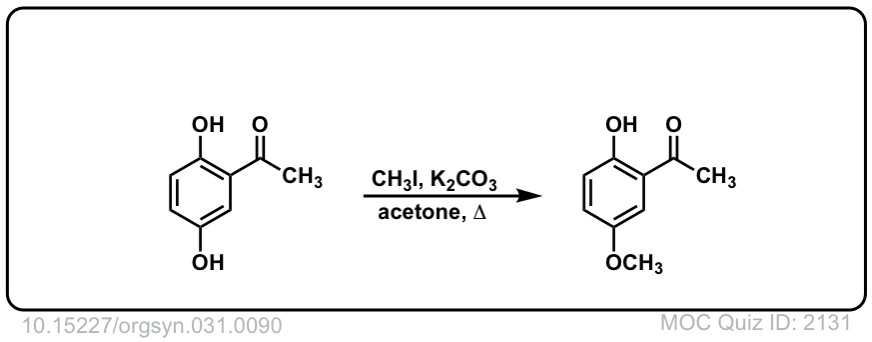
Org. Synth. 1943, 23, 52
DOI Link: 10.15227/orgsyn.023.0052
The ether oxygen should have a dash instead of wedge, isn’t it, as in SN2 inversion occurs!
Does this reaction form alkenes in case elimination takes place?
It certainly could, especially with secondary alkyl halides! I tried to highlight the formation of the ether in the case of the secondary alkyl halide but I should note that I’m not sure on the yield.
why wouldn’t e1 happen with naome and a secondary bromine group on an alkane chain? like why would this proceed with substitution if naome can also be an unhindered base?
E1 is not an option with a secondary bromine. Loss to give a carbocation would not happen without significant heat. On the other hand, with a secondary bromine, E2 is a real issue to watch out for. The substitution reaction would likely be accompanied by elimination products (from E2)
What if we use allyl bromides
Allyl bromides work really well!
E2 elimination is favoured when the atacking alkoxideion is tertiary but in willamsons’ Synthesis sn2 rxn is favoured in presence of tertiary alkoxide ion how is it possible reply me soooon
In the example above the alkyl halide is CH3I and elimination is not possible.
in the last example (a alkyl group attached to the benzene ) the approach of nucleophile is heavily hindered so how would the reaction proceed to the final product ??
It’s not really that hindered. The benzene is flat. Also, benzylic positions are unusually reactive because the aromatic pi system stabilizes partial positive charge in the transition state.
Can you show me the rxn if I use second halide with sodium alkoxide as I want to make an alkene out of it but I want to know the rxn please
use a strong base, preferably alcoholic KOH
How would I figure out which reaction would give the higher yield?
Think about whether you’d want to do an SN2 on a primary alkyl halide or on a tertiary alkyl halide.
why doesn’t E2 occur instead in example 2?
It will likely occur. I’ve just shown the example if a Williamson were to occur.
I have one question. What will be the order of reactivity of the following nuclophiles with methyl chloride:
(A) H3C-O^- Na+
(B) H5C2-O^- Na+
(C) (H3C)2-CH-O^- Na+
(D) (H3C)3-C-O^- Na+
What is the key variable that is changing as you go from H3C-O(-) Na+ to (CH3)3-CO(-) Na(+) ? What do you think will be the mechanism of the reaction? What types of factors are those reactions sensitive to?
Hi, great work!
I have a small doubt, though. If the alkyl halide is tertiary, the reaction will follow SN1 mechanism instead of SN2, and thus a carbocation will be formed.
Q1) Will the reaction not proceed to give us an ether?
Q2) My teacher’s answer to Q1 was no. He said that a carbocation always reacts with a base to give an alkene. Is that actually true?
Thanks in advance!
your teacher is right and that mechanism is E2
great work. would you like to tell ever it work tertiary substrate
Tertiary alkoxide with a primary or methyl halide, yes. Tertiary halide, never.
Can it perform elimination reactions?
With an alkoxide and a secondary alkyl halide, elimination reactions (E2) will be very common.
If you’re starting with an ether and trying to determine which starting materials are required, does it matter which halide you use for the leaving group?
It absolutely matters. Since the reaction is SN2, you want to set the reaction up so that your alkyl halide is (preferably) primary. You certainly wouldn’t want to try to get an SN2 to work on a tertiary alkyl bromide.
Is OH- an alkoxide ion?
No, it’s a hydroxide ion. CH3O- is an example of an alkoxide ion.
He doesn’t need to explain why it’s SN2 :)
When the halide group leaves, if the alkoxy ion doesn’t atake at the same time, you would be left with a primary carbo-cation. These are very unstable because you only have a carbon giving charge to the primary. If you had two more carbons linked to the carbon with the halide, you could have a SN1 reaction, since the carbons around the tertiary carbo-cation would be estabilizing the positive charge.
I hope I made myself clear. I’m Portuguese :)
u dnt explain why the reaction is taking place in sn2 manner.
True, although that’s a rather large subject. Here, note that it works best for primary (and to some extent secondary) alkyl halides.
In a reaction between benzyl chloride and alkoxide although its a primary halide but the carbocation formed(if reaction takes place in Sn1 manner ) is highly stable as it is resonance stabilised benzyllic carbocation , but even in such cases we say its Sn2. Why is that so?
In the absence of a Lewis acid, ionization of the benzylic chloride will be relatively slow. Half life much greater than several hours at room temperature. You didn’t specify solvent, but for example if you leave PMBCl out on the bench overnight, it goes to crap due to this process.
However, if an alkoxide (negatively charged oxygen) is added to the benzyl chloride the direct reaction of the nucleophile with the primary halide will be much, much faster. Reaction within minutes.