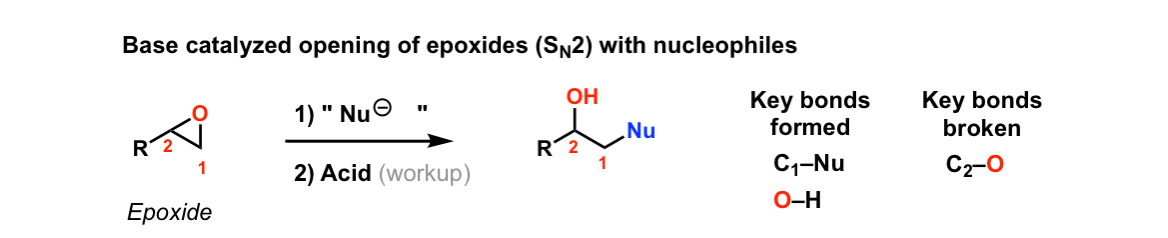
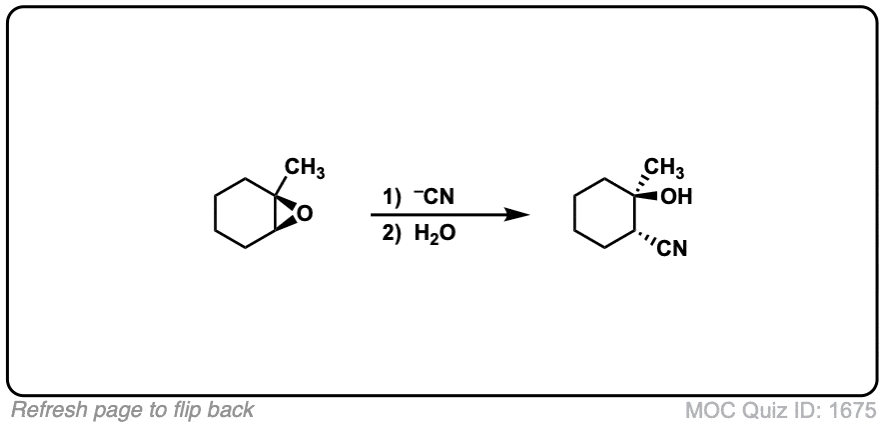
Reaction of epoxides with nucleophiles under basic conditions
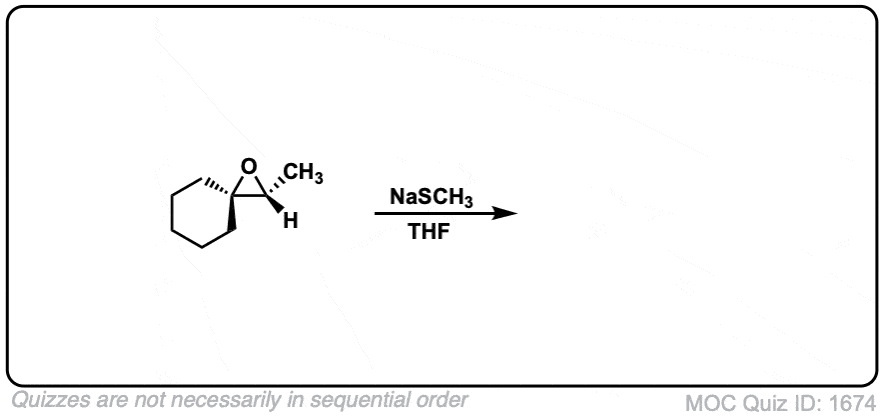
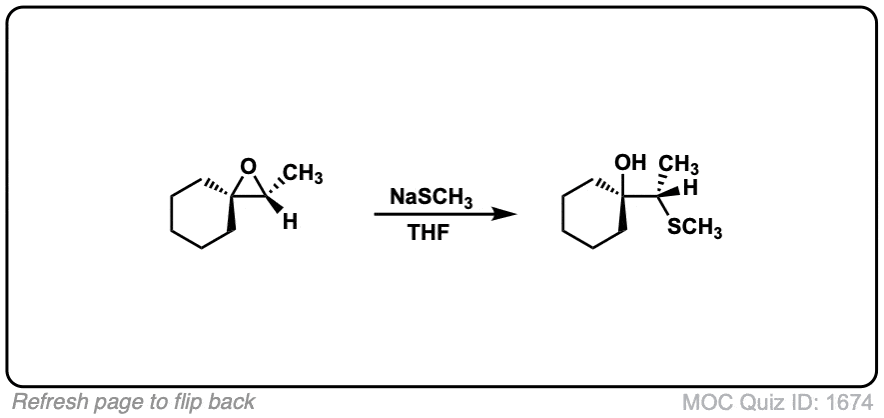
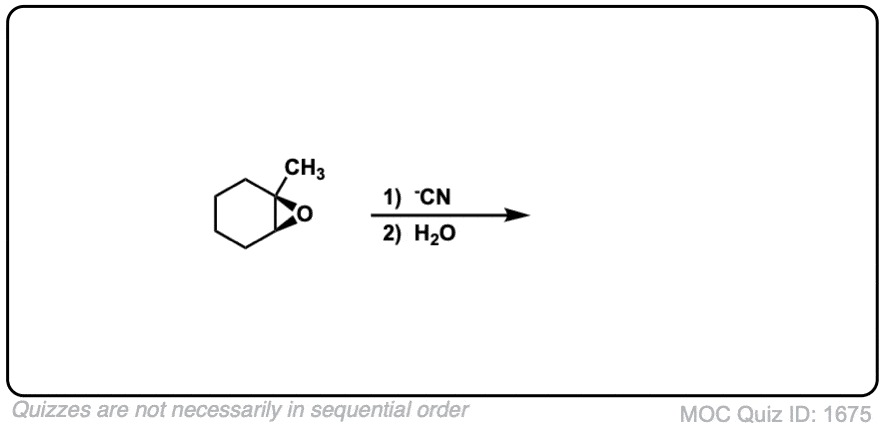
Description: When epoxides are treated with negatively charged nucleophiles, they will add to the least substituted position of the epoxide in an SN2 reaction.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Notes: Recall that epoxides are unlike normal ethers in that they have considerable ring strain due to the 3 membered ring.
- The purpose of the acid in the second step is to protonate the negatively charged oxygen that is formed when the epoxide is opened. This results in formation of a neutral alcohol.
- Where addition occurs to a stereocenter, the stereochemistry of the carbon will invert.
- Some examples of nucleophiles that will add to epoxides include hydroxyl (HO-) alkoxy (RO-), Grignard reagents and (sometimes) organocuprates (Gilman reagents)
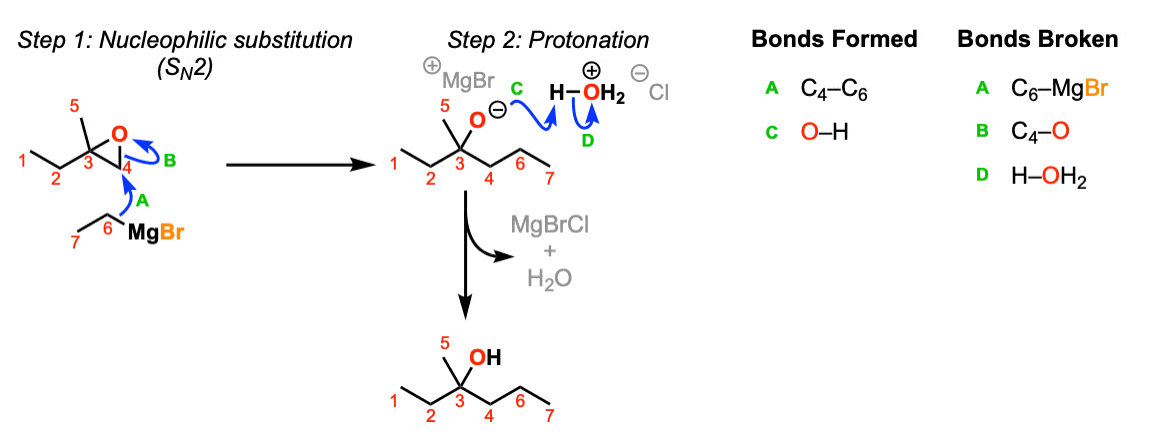
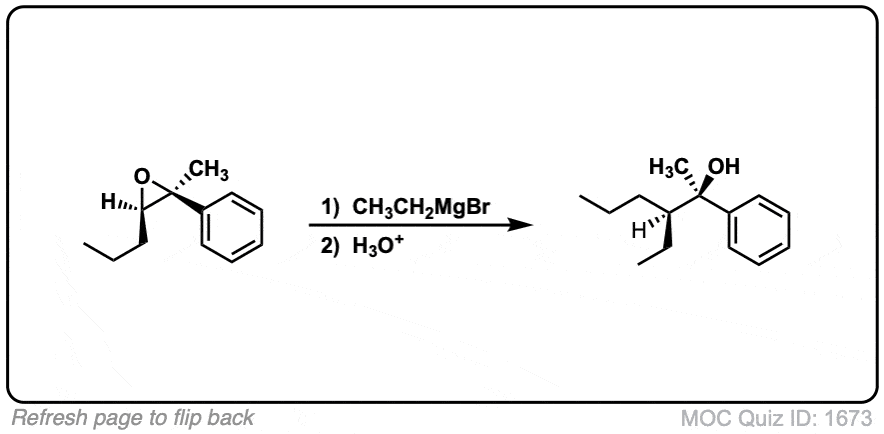
Notes: This depicts opening of an epoxide by a Grignard reagent. The second step is protonation of the oxygen with H3O(+).
Note that there is nothing special about Cl(-) here, it’s just a spectator ion with H3O(+)
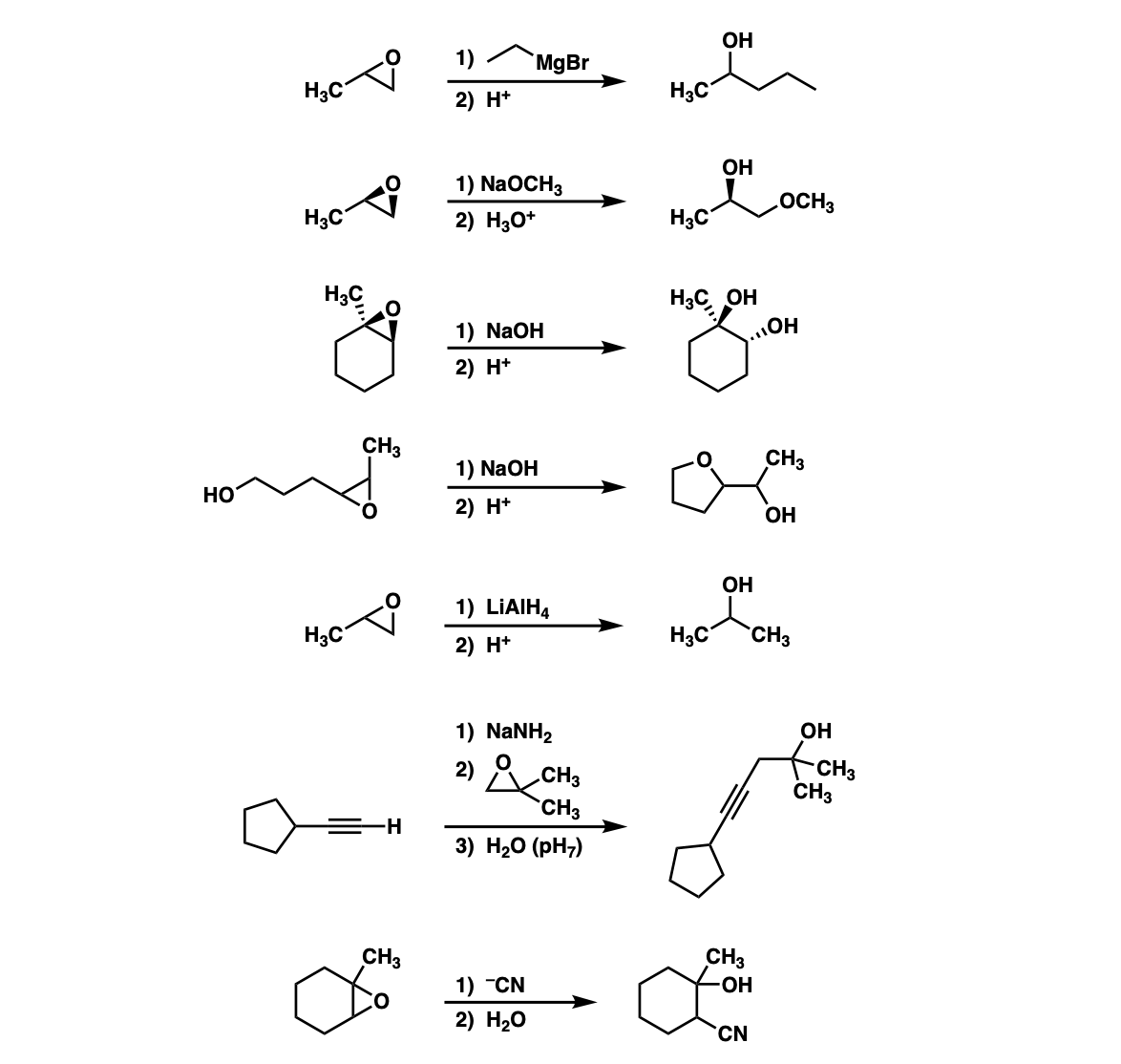
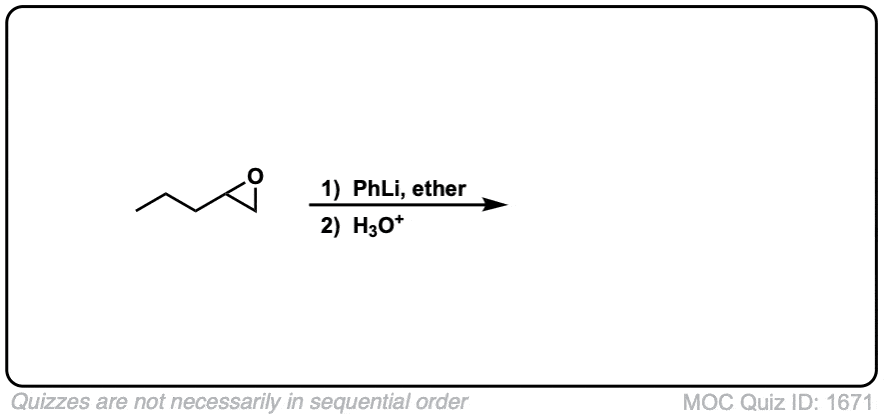
Quiz Yourself:
 Click to Flip
Click to Flip
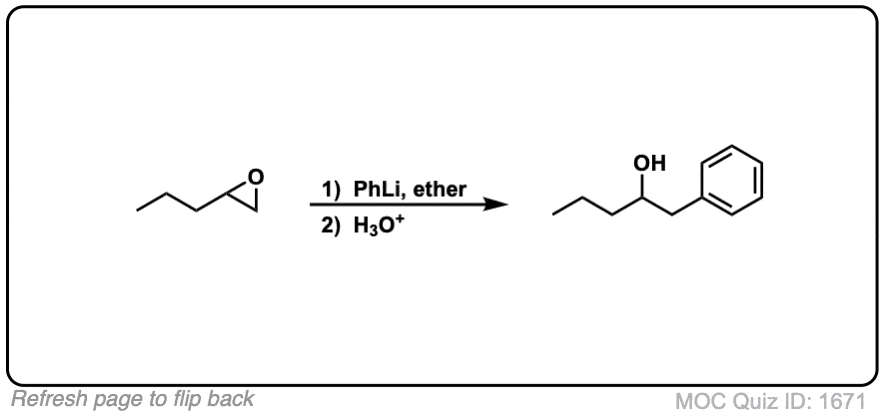
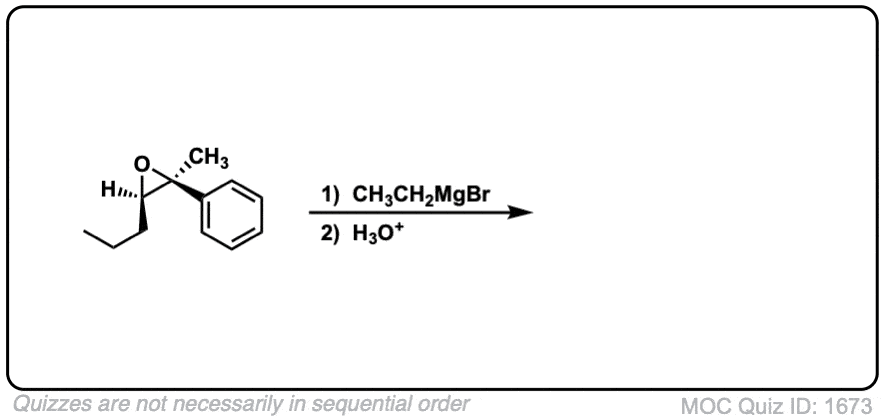
 Click to Flip
Click to Flip
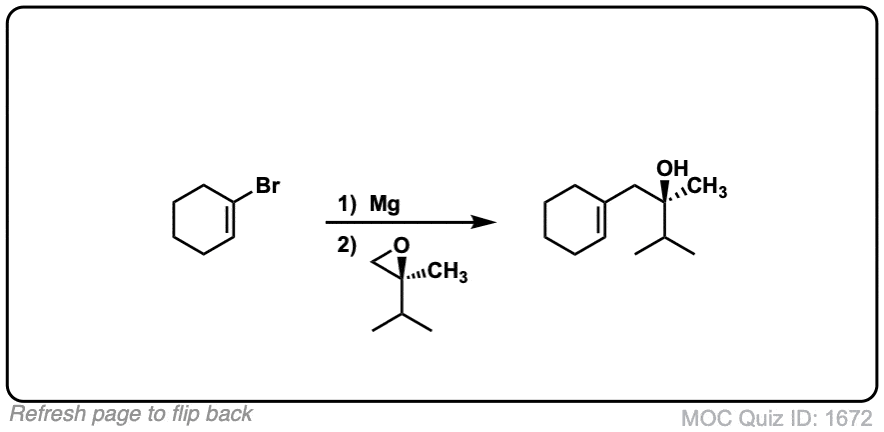
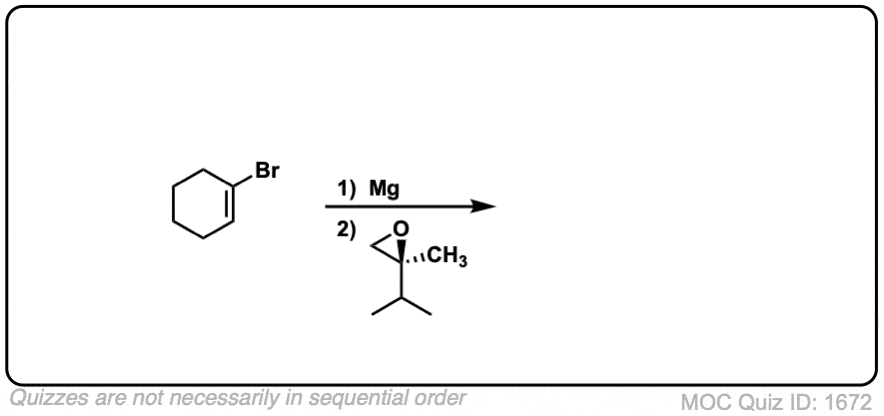
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
(Advanced) References and Further Reading
- Base-Promoted Isomerizations of Epoxides
Crandall, Jack K.; Apparu, Marcel
React. 1983, 29, 345-443
DOI: 10.1002/0471264180.or029.03 - Selective transformations of 2,3-epoxy alcohols and related derivatives. Strategies for nucleophilic attack at carbon-3 or carbon-2
Carl H. Behrens and K. Barry Sharpless
The Journal of Organic Chemistry 1985, 50 (26), 5696-5704
DOI: 10.1021/jo00350a051 - Stereochemistry of the base-induced rearrangement of epoxides to allylic alcohols
Randolph P. Thummel and Bruce Rickborn
Journal of the American Chemical Society 1970, 92 (7), 2064-2067
DOI: 10.1021/ja00710a045 - Organoaluminum reagents of type R1R2NAlEt2 which allow regiospecific isomerization of epoxides to allylic alcohols
Arata Yasuda, Shin Tanaka, Koichiro Oshima, Hisashi Yamamoto, and Hitosi Nozaki
Journal of the American Chemical Society 1974, 96 (20), 6513-6514
DOI: 10.1021/ja00827a044 - Controlled chemical synthesis of the enzymically produced eicosanoids 11-, 12-, and 15-HETE from arachidonic acid and conversion into the corresponding hydroperoxides (HPETE)
E. J. Corey, Anthony Marfat, J. R. Falck, and John O. Albright
Journal of the American Chemical Society 1980, 102 (4), 1433-1435
DOI: 10.1021/ja00524a043 - CYCLOHEXENE IMINE (7-AZA-BICYCLO[4.1.0]HEPTANE)
Iain D. G. Watson, Nicholas Afagh and Andrei K. Yudin
Org. Synth. 2010, 87, 161-169
DOI: 10.15227/orgsyn.087.0161
This is a particularly useful procedure, as it is a Staudinger reaction for converting epoxides to NH-aziridines (the nitrogen analog of epoxides). The synthesis can be tricky, but a lot of detail has been provided here so that this can be reproduced, as fitting a procedure in Organic Syntheses.
Real-Life Example:
Org. Synth. 1926, 6, 54
DOI Link: 10.15227/orgsyn.006.0054
 Click to Flip
Click to Flip
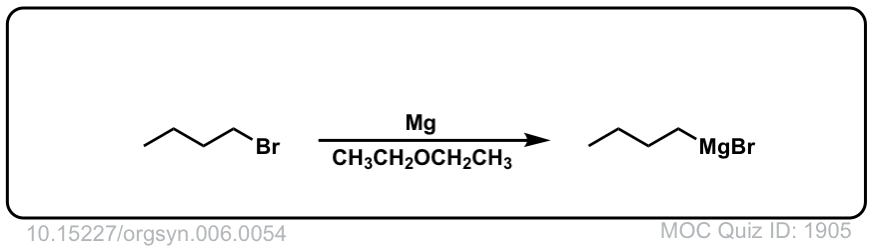
It became MgBrCl in the figure, combining with the counter-ion of H3O+ Cl- . See the curved arrow going off to the side.
Where did the MgBr go?
All examples shown at the acidic conditions. However, the title stated that basic conditions. Is it a mistake or I am missed something?
The nucleophile to open the epoxide is basic (in step 1). This results in a negatively charged oxygen. The purpose of acid workup (step 2) is to protonate the oxygen to give neutral OH.
Could you explain how the ring forms on example 4?
The first step is deprotonation of the alcohol on the far left to give the conjugate base. The conjugate base is a really good nucleophile and attacks the carbon of the epoxide 5 atoms away to make the 5-membered ring. Then, the negatively charged oxygen is protonated with mild acid.
One thing that you’re not often told is that 5 membered ring formation is faster than 6 membered ring formation so it’s unlikely you’d be expected to draw the product given just the starting material, but you would be expected to be able to figure out the mechanism.
Think you could add a page on the opening of epoxides with a Nu to form a Polymer? My text doesn’t show the full mechanism and I would find it really helpful.
In the second stage of the mechanism its should be H-OH2
Thanks a lot . I was wondering how this reaction takes place.