1,4-addition of enolates to enones (“The Michael Reaction”)
Description:
Enolates of carbonyl compounds will add to an α,β-unsaturated carbonyl compounds to give 1,5-dicarbonyl compounds. This is called the Michael reaction.
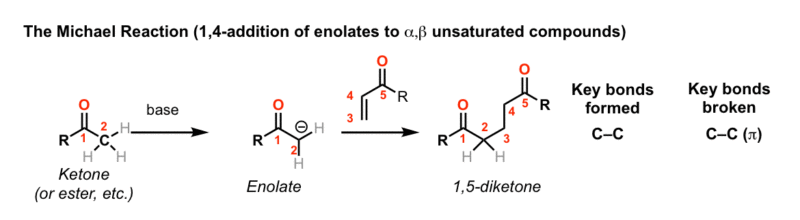
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
I wonder if we can regard Lithium enolate as R-Li and 1,2 addition?
I would not regard a lithium enolate as R-Li, one might confuse a lithium enolate with an organolithium compound. The biggest difference between the two is that in the enolate, the Li is coordinated to the oxygen. Much less basic than the organolithium.
Is there anywhere we can go to see a similar page for 1,2-conjugate addition?
1,2-addition is not conjugate addition. 1,2-addition is just called, “nucleophilic addition to carbonyls”, e.g. https://www.masterorganicchemistry.com/2022/09/09/nucleophilic-addition/
Hi James,
Is not supposed in third example using corresponding base to alkoxide of ester?
Why doesnt the enolate react as an aldol condensation with the unsaturated ketone?
That’s an excellent question. I don’t have a good, short answer for you. It might to some extent, but the aldol addition is reversible. With the Michael, you’re breaking a weaker bond (the C-C pi bond versus the C=O pi bond). On a deeper level there are some molecular orbital considerations to consider (hard-soft acid base theory or HSAB) that we generally don’t get into in intro organic. It’s a deep issue and the answer isn’t immediately obvious.
Hi! to which type of the reaction Sn2 or E2 Aza-Michael reaction refers to?
And which step of the reaction is rate limiting?
Thank you!
It doesn’t refer to SN2 or E2. the Aza-Michael refers to using nitrogen as the nucleophile in a conjugate addition reaction (1,4-addition). See for example the reaction in this abstract. https://pubs.acs.org/doi/abs/10.1021/ol202276h
What would happen if we used an organometallic reagent (R MgX or R2CuLi) as a nucleophile? Would it give us a 1-4 addition product or will it directly attack the carbonyl carbon? Has the nucleophilic strength anything to do with it?
Well, you’ve just so happened to give the two canonical examples of nucleophiles that will add to different segments of the molecule. If you use R2CuLi, addition gives a 1,4 product with 100% addition to the alkene and no addition to the carbonyl. If you use a Grignard reagent, addition gives a 1,2 product directly to the carbonyl. It’s thought that the highly polarized C-Mg bond has a greater partial negative charge on carbon, and this is attracted to the partial positive charge on the carbonyl. With copper, it’s a longer story, but the alkene pi bond can coordinate to the Cu which is followed by addition to the beta carbon.
If 1,4- addition and 1,2- addition pathways are both possible, how do we decide which one the reaction actually goes through?
Hi Neha,
I just commented on the same question above, so I’ll tell you how I remember it, as well.
Here’s a link to James’ explanation of conjugate addition:
https://www.masterorganicchemistry.com/tips/conjugate-addition/
I’m just a student, but anytime I have a conjugated ketone, I have to make a decision between 1,2 direct addition and 1,4 conjugate addition. The direct addition is the kinetic product and the conjugate addition is the more stable thermodynamic product.
1,2 direct addition is only accomplished by “super hero” amazing nucleophiles. These would be nucleophiles like Grignard reagents (MgBr), Hydrides, or ylides like Wittig reagents (PPh3). I’m sure there are more, but in my O-Chem class, those are the only three we have to worry about. If it’s not one of those, it is going to add 1,4.
Enolates are great nucleophiles, but they are not “super hero” nucleophiles. So, they will readily add 1,4.
Hope that helps.
thank you James, the mechanism makes sense now, and the numbering is creally helpful…
Usually more substituted alpha C of michael donar is involved in the addition. Even bulky base using. Is it right?
Pertaining to the third step of the mechanism after the conjugate addition, could the negative charge on the carbon not also be on the oxygen as the lone pair is in conjugation with the carbonyl group? In that case, protonation would give a hydroxy ketone. Is this correct?
Protonation on carbon would just give the ketone, not the hydroxy ketone. Protonation on oxygen would give the enol, which through a process called “tautomerism”, would quickly convert to the ketone.
Hey James! Quick question:I read in Klein’s book that Michael donors have to be an enolate from a di-carbonyl compound (because it’s more stable and less reactive, etc) in order to be selective to strictly undergo 1,4-addition. Is steric hinderance the reason why 1,4-addition prefer to occur in this case?
Many thanks!
Hi Fiona,
Here’s a link to James’ explanation of conjugate addition:
https://www.masterorganicchemistry.com/tips/conjugate-addition/
I’m just a student, but anytime I have a conjugated ketone, I have to make a decision between 1,2 direct addition and 1,4 conjugate addition. The direct addition is the kinetic product and the conjugate addition is the more stable thermodynamic product.
1,2 direct addition is only accomplished by “super hero” amazing nucleophiles. These would be nucleophiles like Grignard reagents (MgBr), Hydrides, or ylides like Wittig reagents (PPh3). I’m sure there are more, but in my O-Chem class, those are the only three we have to worry about. If it’s not one of those, it is going to add 1,4.
Enolates are great nucleophiles, but they are not “super hero” nucleophiles. So, they will readily add 1,4.
Hope that helps.
Thanks for numbering and this makes so much more sense!!!
Thank you this is easy to follow. I actually understand what is going on.
Thanks for the numbering part James. It was driving me mad.
Plus, your tip of always making sure to count my Carbons really helps as well.
It makes a big difference! Mistakes are really easy to make.