1,4-addition of nucleophiles to enones
Description: Alpha beta unsaturated ketones can undergo reaction with a variety of nucleophiles to form 1,4-addition products (aka “Michael products”)
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples
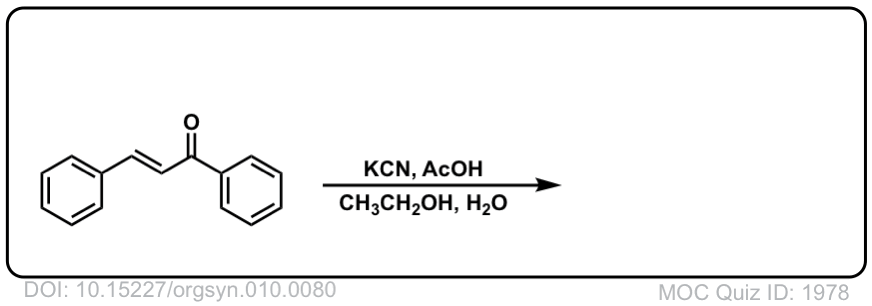
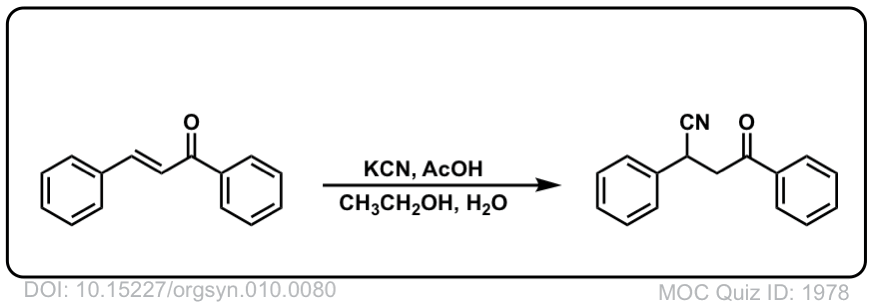
Org. Synth. 1930, 10, 80
DOI Link: 10.15227/orgsyn.010.0080
 Click to Flip
Click to Flip
I am wondering what would happen if we reacted Alpha beta unsaturated ketone and e.g. morpholine? What would act as a nucleophile: oxygen or nitrogen?
Thanks.
With morpholine, only the nitrogen will be nucleophilic in that case. The oxygen would lead to a positively charged species that cannot become neutral through loss of hydrogen. If you had to compare a molecule with an amine and an alcohol, say, ethanolamine, the nitrogen would be more nucleophilic since nucleophilicity of a lone pair increases as you go to the left along the periodic table (N > O)
Thank you for the response. That has cleared things up.
What is the reason for strong nucleophiles favoring 1,2 addition while weak nucleophiles favor 1,4 addition? is 1,2 less sterically hindered (kinetic addition product) but 1,4 thermodynamically favored?
Also, enolates, being weak nucleophiles, usually favor adding 1,4 right?
Great question. It has to do with a topic that doesn’t get much discussion in organic chemistry until later on – called “Hard-soft acid-base theory”. Nucleophiles with a concentrated charge density like organolithiums and Grignards tend to add to the atom of the enone with the highest charge density (the carbonyl carbon). For a full explanation I’d need to invoke molecular orbital theory.
I see. Thanks! Do enolates always prefer to add via 1,4 addition?
Generally speaking, yes.
Good point. It trips a lot of people up. I should write a post and link to it.
Could you please explain some of the terminology that is commonly used for carbonyl chemistry – alpha beta, and especially 1,4. And provide suggestions on keeping things sorted in memory. Or provide a link if you have already done so.
The numbering particularly threw me when I was a student. We had just become comfortable numbering carbons for nomenclature and here comes carbonyl chemistry to derail us.