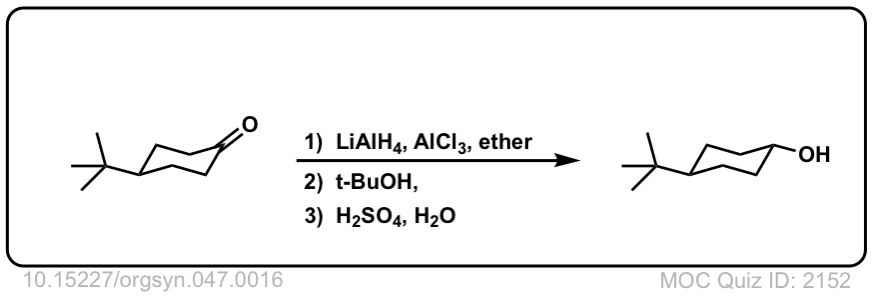
Addition of LiAlH4 to ketones to give secondary alcohols
Description: Addition of lithium aluminum hydride to ketones leads to formation of secondary alcohols (after addition of acid)
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
Org. Synth. 1967, 47, 16
DOI Link: 10.15227/orgsyn.047.0016
 Click to Flip
Click to Flip
I’m curious about something. In the protonation step, where does the (seemingly) H3O come from that protonates the O^-? The diagram says we are using H2SO4/H20 so does the H3O happen from the H2SO4/H2O interaction? I’m sorry if this is a stupid question.
yes
The Al starts in the -5 oxidation state with [Ar] valence. It prefers +3 [Ne] valence configuration. It pushes off an H- first. That extra electron ends up breaking one of the bonds on the protonated oxygen giving O- which then immediately bonds with the proton (H+). From a charge accounting perspective we have a leftover Li+. The Li+ goes with the HS04- I *think* because the sulfate anion is going to more eletronegative and thus LiSO4 is a lower energy state than if it displaced the hydrogen on the newly formed OH group.
I have one question ?
Cyclohexadioenone (E) is extermely prone to tautomersim.Why?
Try drawing the tautomer. What do you notice?
On stereochemistry – Since this is a flat structure before AlH3, does this produce equal amounts of enantiomers if there is a stereocenter at the Carbon?
LiAlH4 seems to function in the same alcohol reduction reactions as the reagent, NaBH4. If LiAlH4 achieves the same products as NaBH4, what is the point of having to remember NaBH4 as a reagent?
NaBH4 is a milder reducing agent and is able to be used in protic solvents. It will only reduce the aldehyde or ketone. LAH is a very strong reducing agent and cannot be used in protic solvents due to a violent deprotonation releasing H2 gas. Therefore it must be used separate to the aqueous work up.
Yes, thanks for your answer, Camden.
NaBH4 reacts only with aldeydes and ketones. LiAlH4 reacts with aldehyde, ketone, carboxylic acid, acid chloride and esters
You are correct, Gabriel.
NaBH4 is for selective reduction. For example, if one had a molecule in which a ketone and a carboxylic acid were present and the carboxylic acid was wanted in the final product, one would use NaBH4 because it cannot reduce carboxylic acids (or any derivatives). LiAlH4 is a much stronger reducing agent and would simply convert both of those groups to secondary alcohols.
I hope this helps!
-Brian