Baeyer-Villiger Reaction
Description: Treatment of an aldehyde or ketone with a peroxyacid (RCO3H) results in the formation of an ester.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
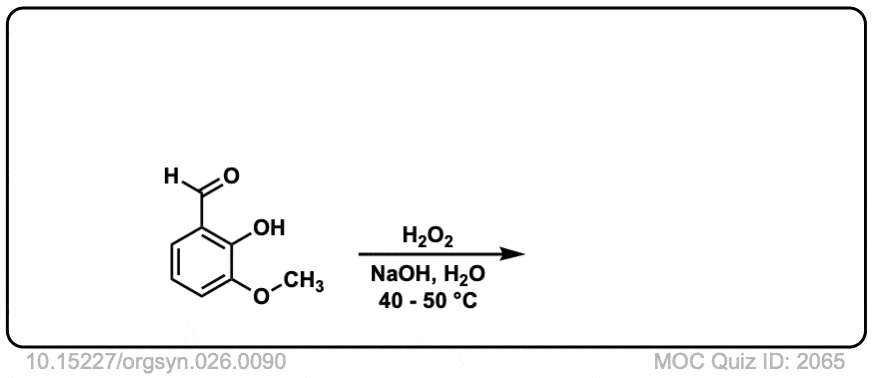
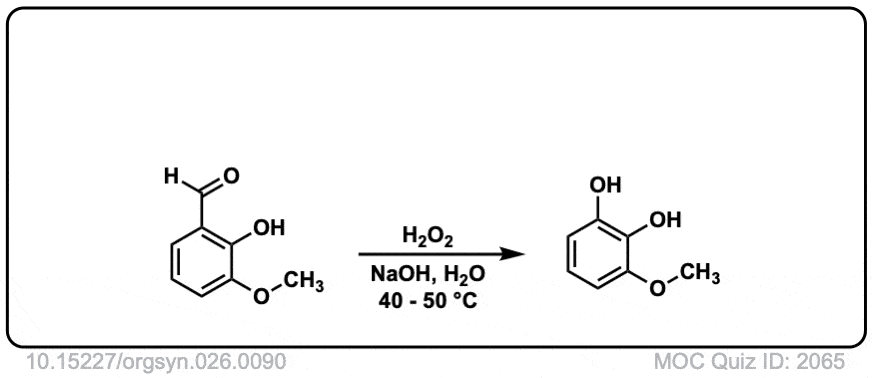
Org. Synth. 1946, 26, 90
DOI Link: 10.15227/orgsyn.026.0090
 Click to Flip
Click to Flip
Hi, is there a mechanism for each reaction? I’m having a hard time
Hi, the last example is an aldehyde. What happens here is that the peroxyacid adds to the aldehyde, and then the aromatic ring migrates to the O, with the O-O bond breaking.
Technically this is referred to as Dakin oxidation (don’t get hung up on the name) but there is a wikipedia page with full mechanism in this case:
https://en.wikipedia.org/wiki/Dakin_oxidation#:~:text=The%20Dakin%20oxidation%20(or%20Dakin,a%20benzenediol%20and%20a%20carboxylate.
Hey, can you please explain the last example?
Yes – that is absolutely right.
So when it reacts with an aldehyde it will result in a carboxylic acid, with another carboxylic acid as a by product?