Claisen Condensation of esters
Description: When esters are treated with strong base, an enolate can be formed. The resulting enolate can add to another ester, resulting in a substitution at the carbonyl carbon.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
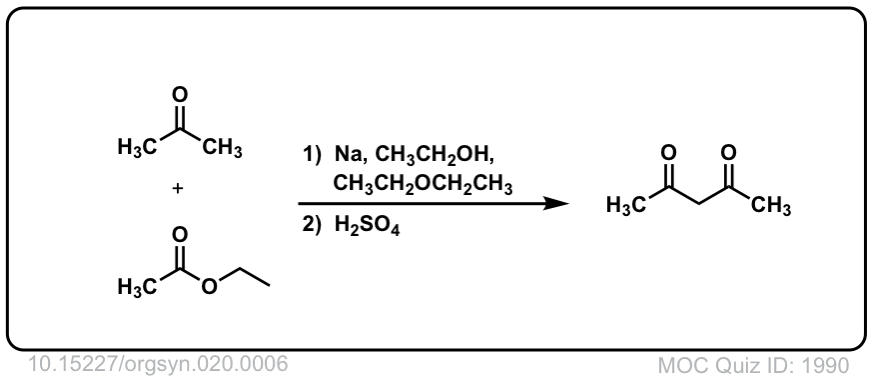
Org. Synth. 1940, 20, 6
DOI Link:10.15227/orgsyn.020.0006
 Click to Flip
Click to Flip
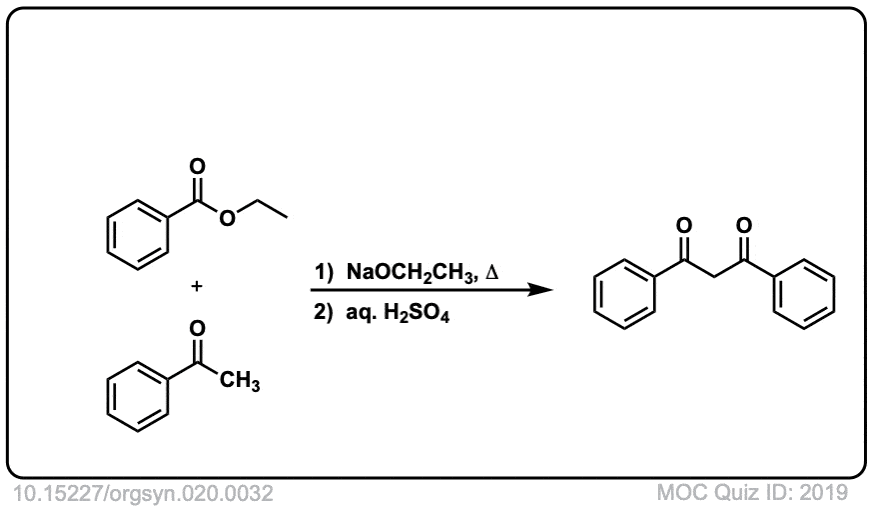
Org. Synth. 1940, 20, 32
DOI Link: 10.15227/orgsyn.020.0032
 Click to Flip
Click to Flip
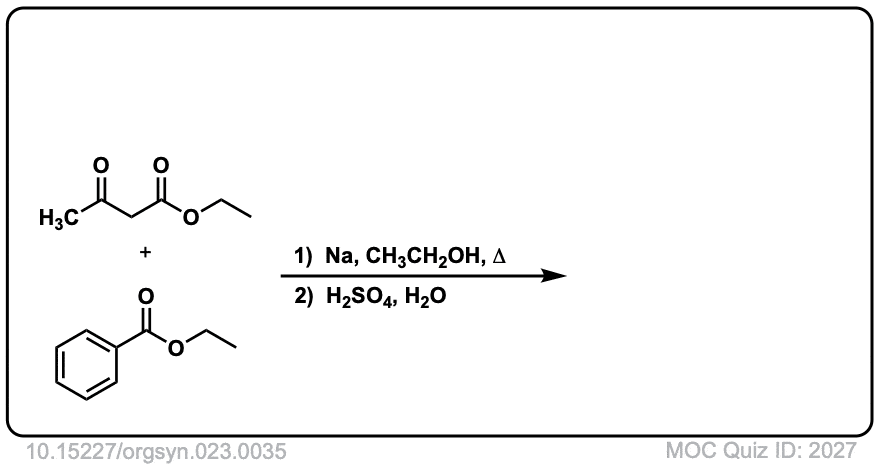
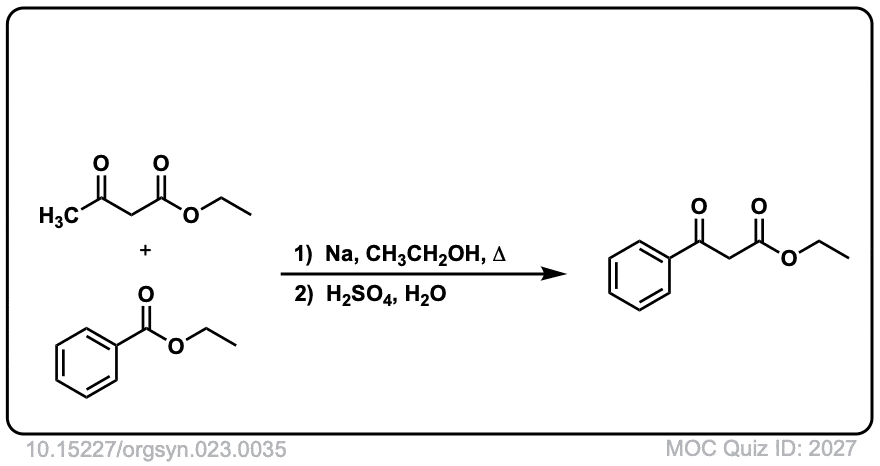
Org. Synth. 1943, 23, 35
DOI Link: 10.15227/orgsyn.023.0035
 Click to Flip
Click to Flip
Hi! I did not quite understand the mechanism of the 6th example given above, the Dieckmann reaction…can someone pls explain? I think I’ve understood the first part where the cyclic ester reacts with alcohol to open the ring but then, I’m a bit confused….
Hi! I did not quite understand the mechanism of the 6th example given above, the Dieckmann reaction…can someone pls explain?
I think I’ve understood the first part where the cyclic ester reacts with alcohol to open the ring but then, I’m a bit confused….
The enolate of carbon 6 attacks carbon 1. This forms a six-membered ring. Then the oxygen attached to carbon 4 is eliminated.
why hydroxide ion (HO–) would lead to hydrolysis of the ester?
is not supposed formation of enolate is acid base reaction and it is faster from addition reaction?
Two issues. 1) The choice of base is usually the conjugate base of the solvent. Hydroxide ion is the conjugate base of water. Water is not going to dissolve most substrates you want to use in this reaction.
2) Even if you use, say, 1-2 equivalents of KOH in ethanol or methanol in solvent, equilibrium will result in formation of KOR as the active base since it’s present in vast excess.
3) Acid-base reactions on heteroatoms (O-H, N-H, S-H) tend to be faster than addition reactions. Not sure I’d say the same applies with formation of an enolate, which does require significant reorganization of the molecule (i.e. rehybridization of the alpha carbon).
4) Why take the chance? You’re putting your time, effort and energy into a procedure. Why take even the slightest chance in decreasing your yield, when it costs you basically nothing to use alkoxide as a base instead of KOH?
Ok, Thank you so much for this explanation
Can you also go over the reformatsky reaction?
Since the b-keto ester has an acidic proton, does the reaction of ester with base need to be followed by a work-up step?
In practice, yes, one would probably use a mild acid like NH4Cl or citric acid in the workup.
is there any possibility of dickmann reaction if -NH- group present in molecule?
i think -NH- group give anion instead of -CH2-.
Some of these are reversible right? (the ones which have only 1 alpha H?)
I believe the reversibility depends on the type of base you are using and not the number of the alpha hydrogens.
Here are some common bases for the irreversible forms:
NaH, NaNH2, KH, LDA
Common reversible bases:
NaOEt, NaOMe