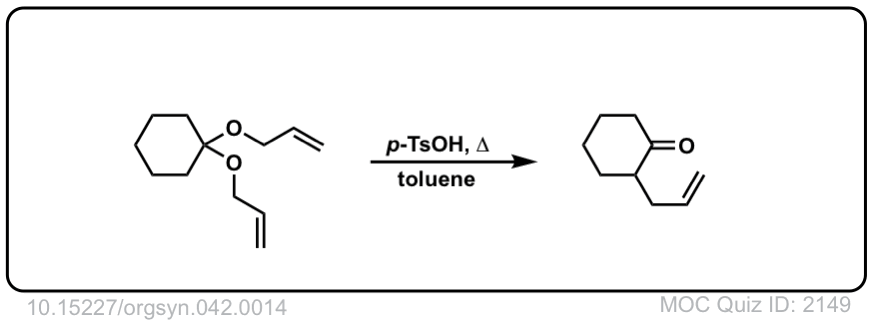
Claisen Rearrangement
Description: The Claisen Rearrangement is a [3,3]-sigmatropic rearrangement of allyl vinyl ethers. It results in a gamma,delta-unsaturated carbonyl.
Notes: The product of this reaction is more stable than the reactant by about 20 kcal/mol, owing mostly to the greater strength of C-O (pi) bonds (80 kcal/mol) versus C-C (pi) bonds (60 kcal/mol).
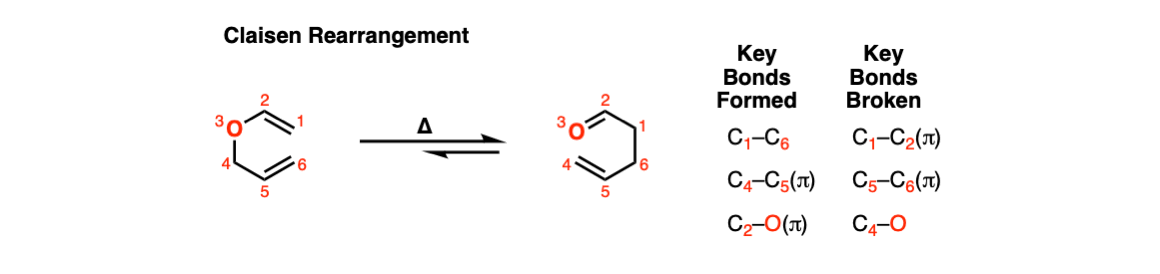
Examples:
Notes: In the first two examples, heating results in the formation of a gamma,delta-unsaturated carbonyl.
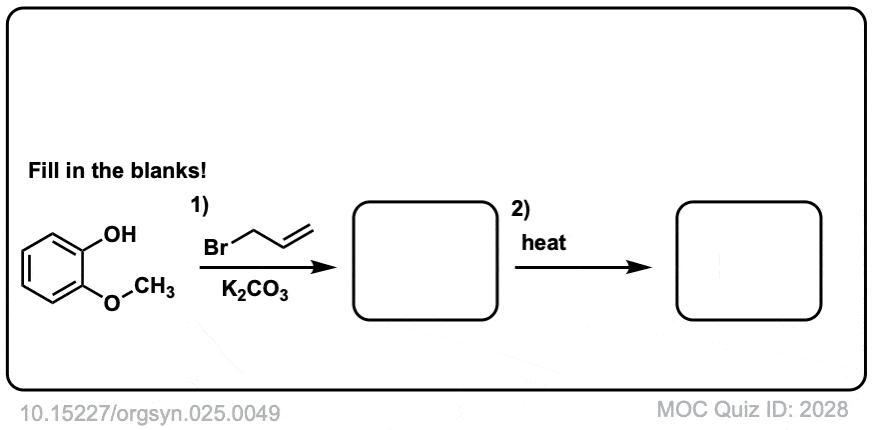
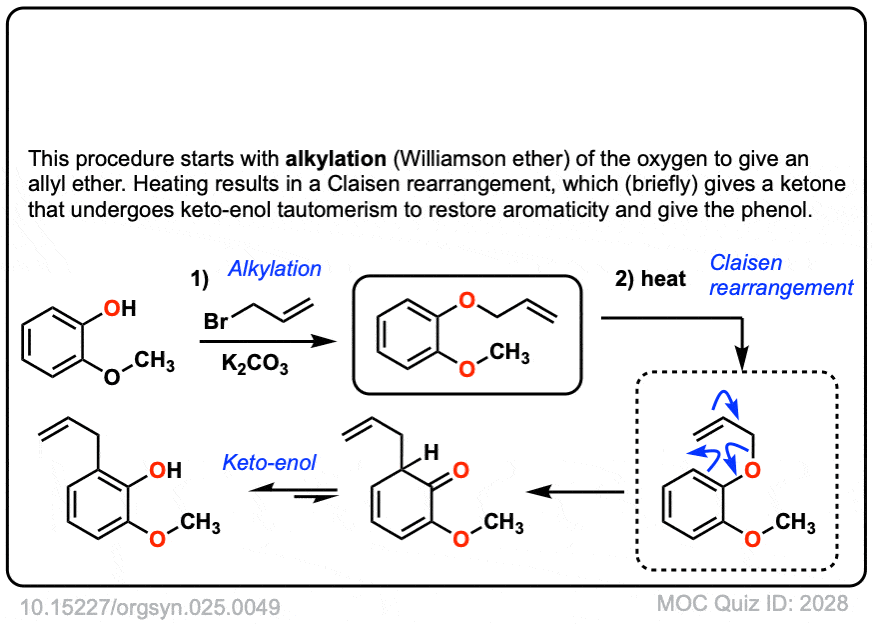
The third example involves the allyl ether of a phenol. In this case a pi bond from the aromatic ring acts as the “vinyl ether” component. The first product is a gamma,delta-unsaturated carbonyl, which is not aromatic. This then undergoes keto-enol tautomerism to restore aromaticity.
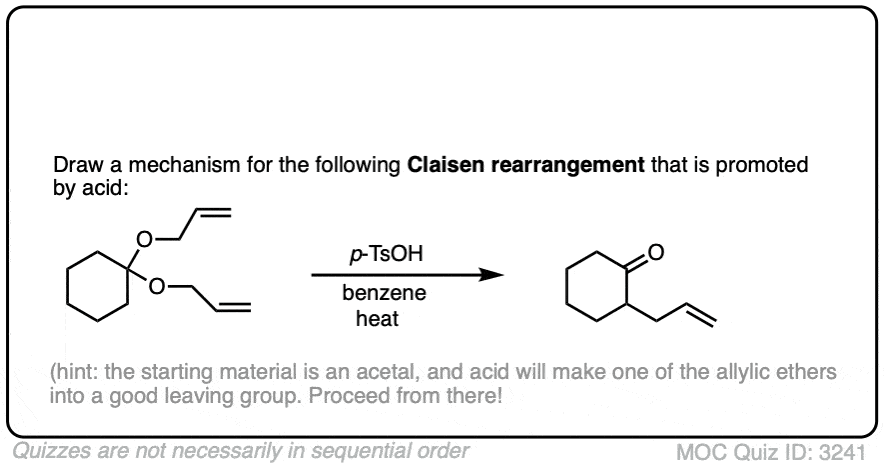
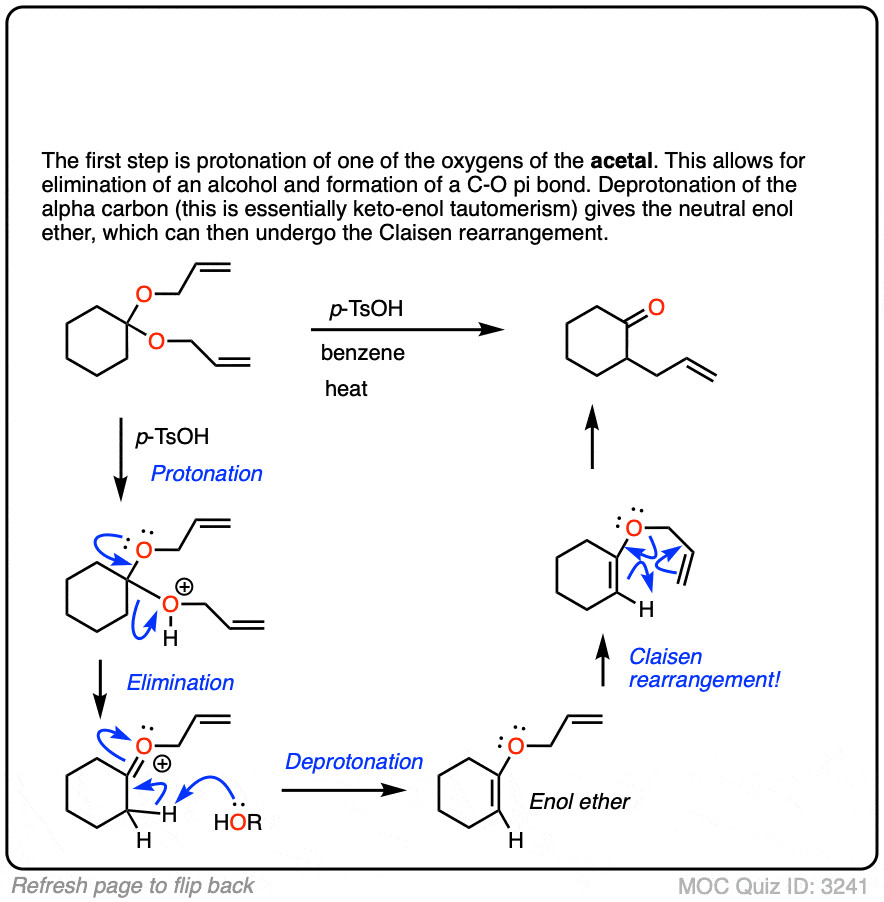
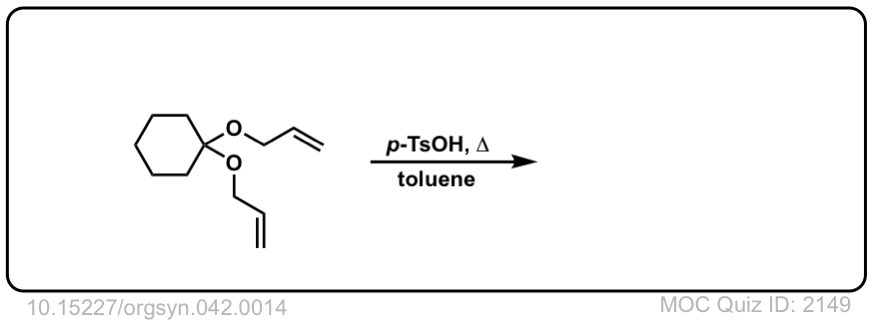
The fourth example is somewhat tricky. Acid (TsOH) protonates one of the acetal oxygens, which sets it up as a good leaving group. The lone pair of the other acetal oxygen forms a new pi bond to carbon, resulting in elimination of one equivalent of allyl ether. This new species is an oxonium ion (positively charged oxygen) which then loses a proton to form an enol ether (much like in the formation of enamines). The resulting vinyl allyl ether then undergoes the Claisen rearrangement, giving the product shown. See the quiz below!
The fifth example is also tricky! The first step is a Claisen rearrangement to install an allyl group at the ortho position. However this cannot lose hydrogen to restore aromaticity since there is a CH3 there! Instead, a Cope rearrangement occurs at the para– position to give the final product.
Mechanism:
The first (and only) step of the Claisen rearrangement is the concerted pericyclic reaction of the vinyl allyl ether, going through a cyclic transition state (Step 1, arrows A, B and C).
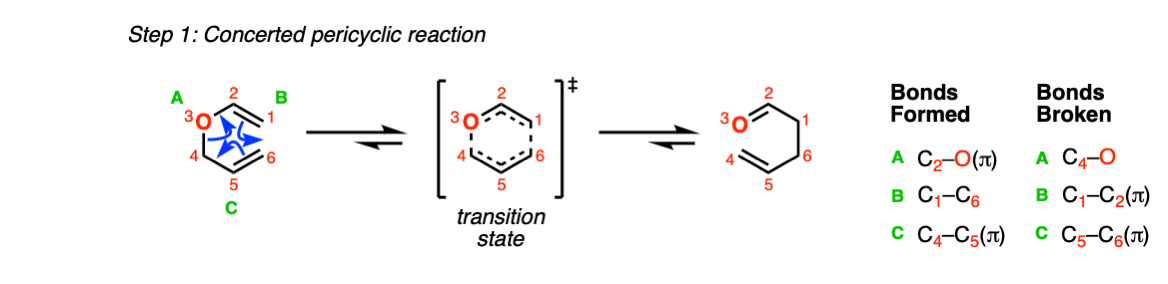
Notes:
We can calculate the driving force of the reaction by comparing the strength of the bonds being broken and formed.
Bonds broken: C-C pi (65 kcal/mol) and C-O sigma (85 kcal/mol)
Bonds formed: C-O pi (85 kcal/mol) and C-O sigma (85 kcal/mol).
Net: 20 kcal/mol which renders the reaction essentially irreversible.
See also: The Cope and Claisen Rearrangements
 Click to Flip
Click to Flip
Real-Life Examples:
Org. Synth. 1945, 25, 49
DOI Link: 10.15227/orgsyn.025.0049
 Click to Flip
Click to Flip
Org. Synth. 1962, 42, 14
DOI Link: 10.15227/orgsyn.042.0014
 Click to Flip
Click to Flip