Conversion of alcohols to alkyl bromides using PBr3
Description: Treatment of a primary or secondary alcohol with PBr3 results in alkyl bromides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
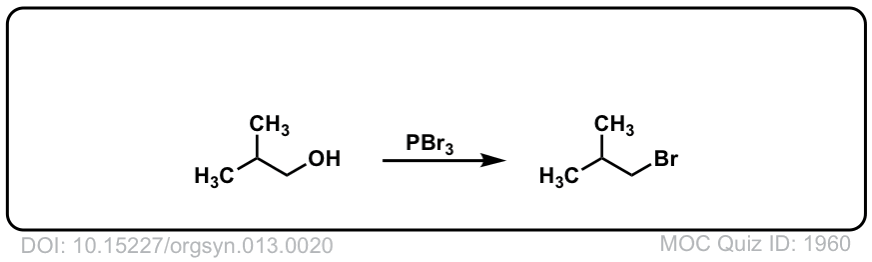
Org. Synth. 1933, 13, 20
DOI Link: 10.15227/orgsyn.013.0020
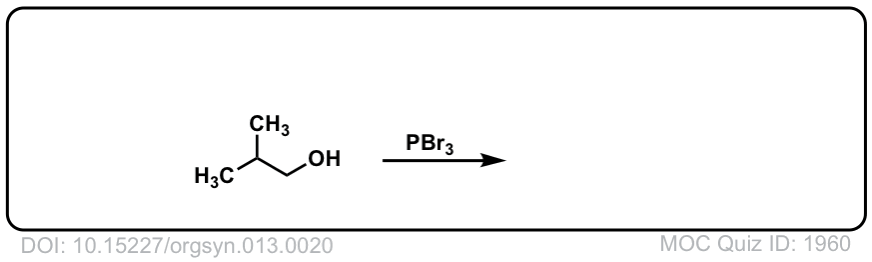 Click to Flip
Click to Flip
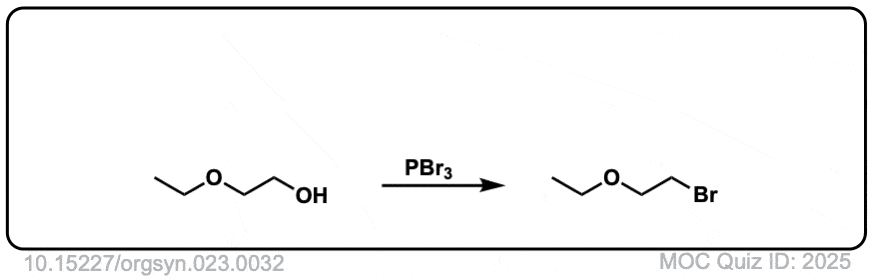
Org. Synth. 1943, 23, 32
DOI Link: 10.15227/orgsyn.023.0032
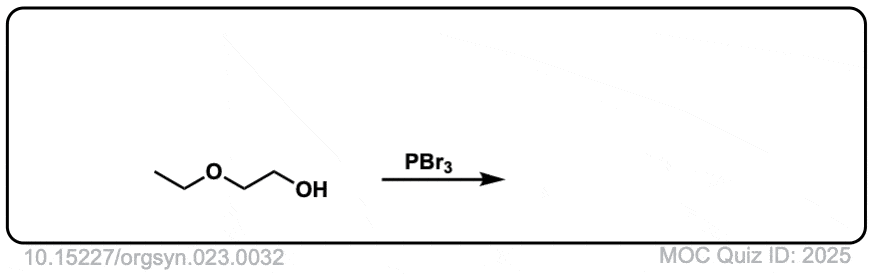 Click to Flip
Click to Flip
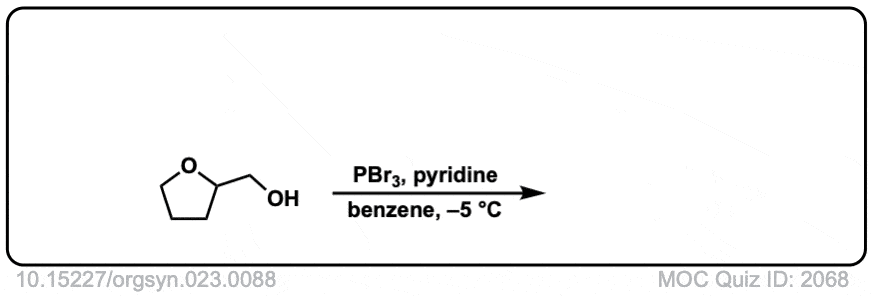
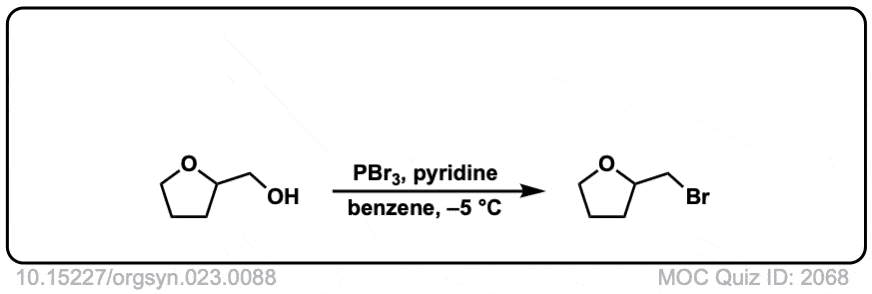
Org. Synth. 1943, 23, 88
DOI Link: 10.15227/orgsyn.023.0088
 Click to Flip
Click to Flip
Is there a particular solvent that this reaction is carried out in? My organic chemistry lecture states that it’s carried out in ethers sometimes. What is the reasoning behind this?
Pretty standard. Ethers like THF, diethyl ether will work fine, as will halogenated solvents like CH2CL2.
It can be done with the alcohol as solvent, if on large enough scale. 1) http://www.orgsyn.org/Result.aspx?ga=na 2) http://www.orgsyn.org/Result.aspx?ga=na
EDIT: Also benzene, with added pyridine. http://www.orgsyn.org/Result.aspx?ga=na
Is the reaction mechanism the same for an alcohol and PCl5?
Very similar, yes!
will this reaction occur if it’s a tertiary alcohol?
I don’t believe so due to the fact that the reaction proceeds through SN2. In order to convert a tertiary alcohol into an alkyl halide, it is better to use SOCl2 or SOBr2. Correct me if I am wrong.
Your reaction with PBr3 and the 2ndary alcohol is incorrect, I believe. Shouldn’t that be a Br instead of an OH in the product?
Yes, thanks for pointing out the error! fixed!
this is most useful specially for the student of class xi &xii