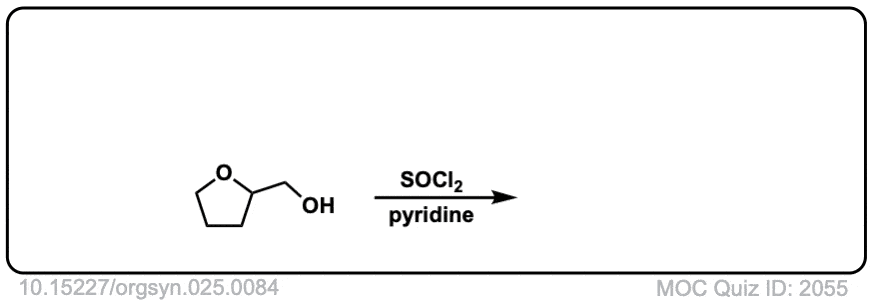
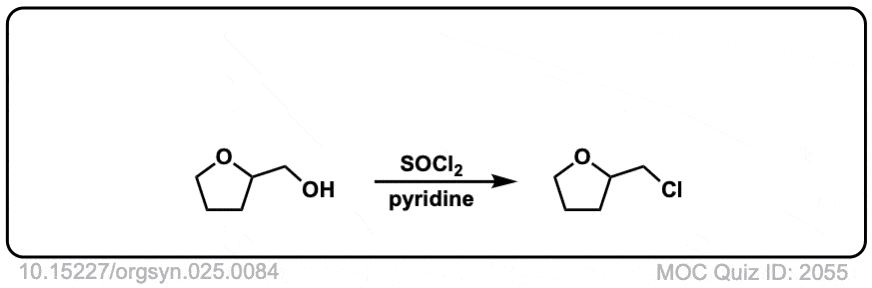
Conversion of alcohols to alkyl chlorides using SOCl2
Description: Primary or secondary alcohols can be converted into alkyl chlorides through treatment with SOCl2.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1945, 25, 84
DOI Link: 10.15227/orgsyn.025.0084
 Click to Flip
Click to Flip
Hello 🙂, I wonder why chloride ion attacks carbon in step 3 in the mechanism of this reaction, while in step 3 in the mechanism of formation of tosylates from alcohols chloride ion attacks H? thanks.
Hi,
why is this reaction usually carried out in pyridine or a tertiary amine?
The purpose of the tertiary amine is to neutralize any HCl that is formed during the reaction. [Pyridine can also displace Cl from SOCl2 to give SOCl(pyr+) which has a better leaving group, but that’s a detail I didn’t want to go into].