Decarboxylation of beta-keto carboxylic acids
Description: When carboxylic acids containing a carbonyl group two bonds away (“on the β carbon”) are heated, carbon dioxide is lost.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples
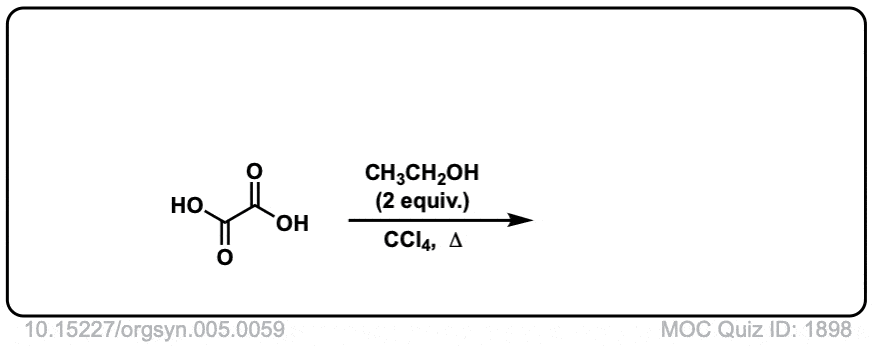
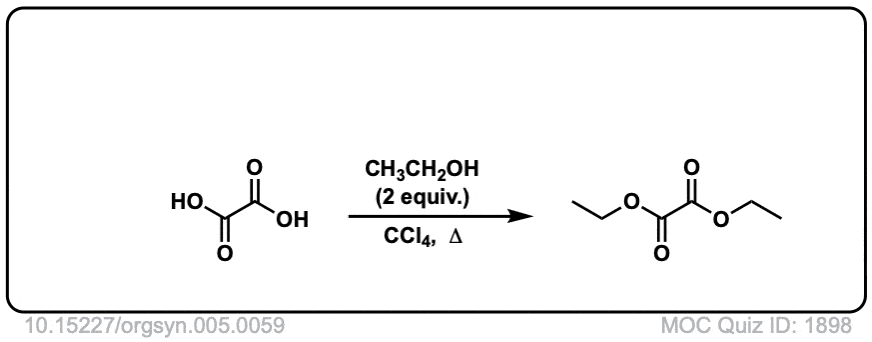
Org. Synth. 1927, 7, 40
DOI Link: 10.15227/orgsyn.007.0040
 Click to Flip
Click to Flip
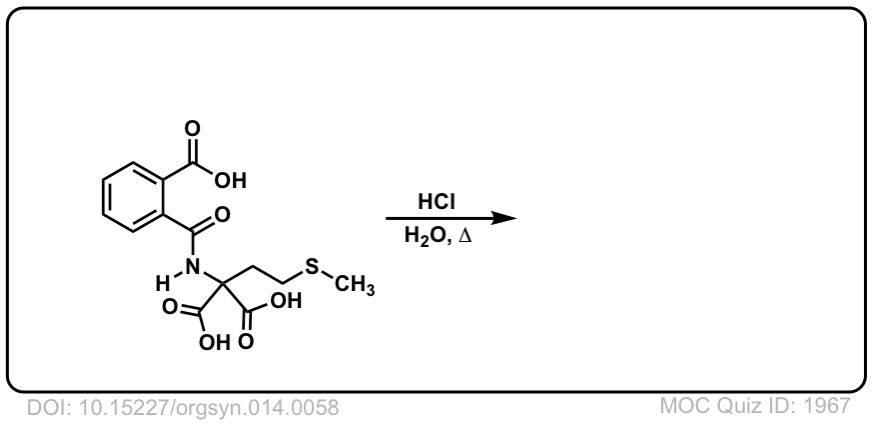
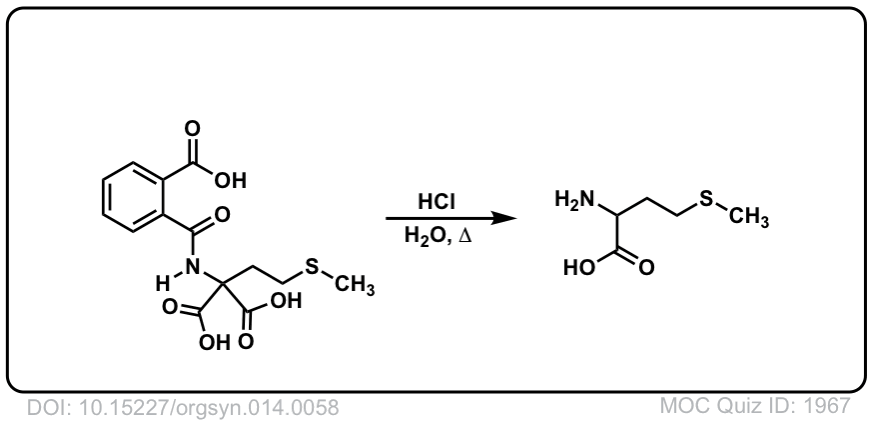
Org. Synth. 1934, 14, 58
DOI Link: 10.15227/orgsyn.014.0058
 Click to Flip
Click to Flip
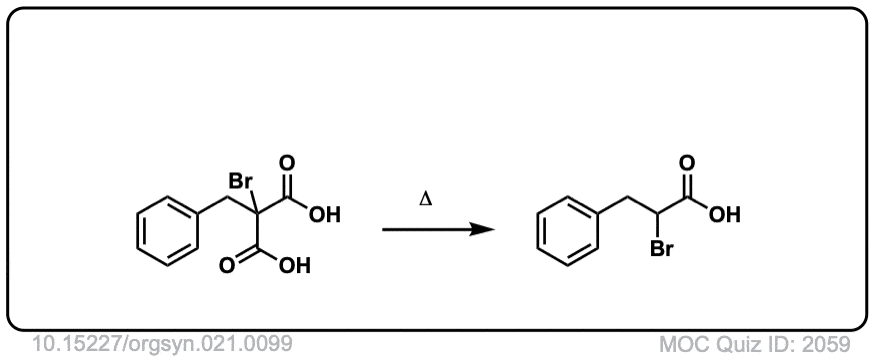
Org. Synth. 1941, 21, 99
DOI Link: 10.15227/orgsyn.021.0099
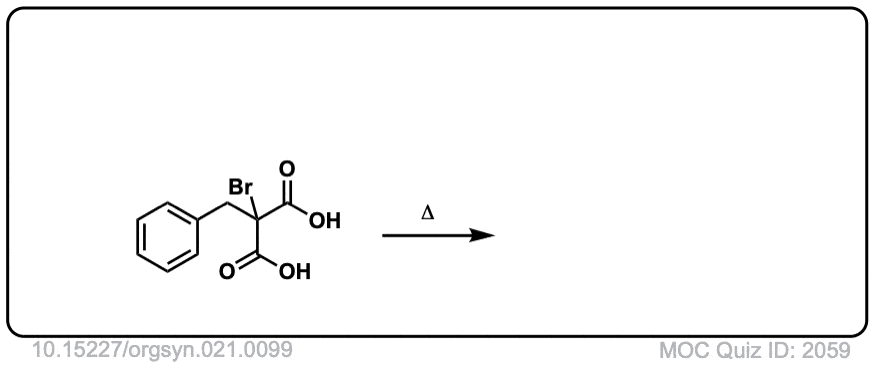 Click to Flip
Click to Flip
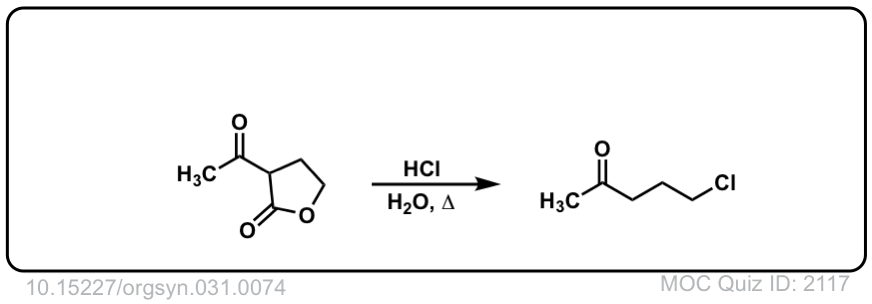
Org. Synth. 1951, 31, 74
DOI Link: 10.15227/orgsyn.031.0074
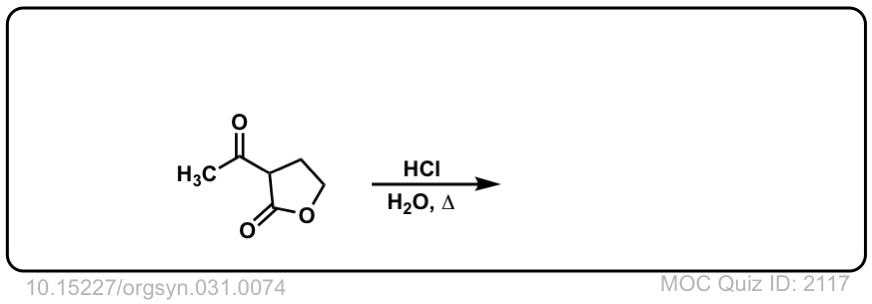 Click to Flip
Click to Flip
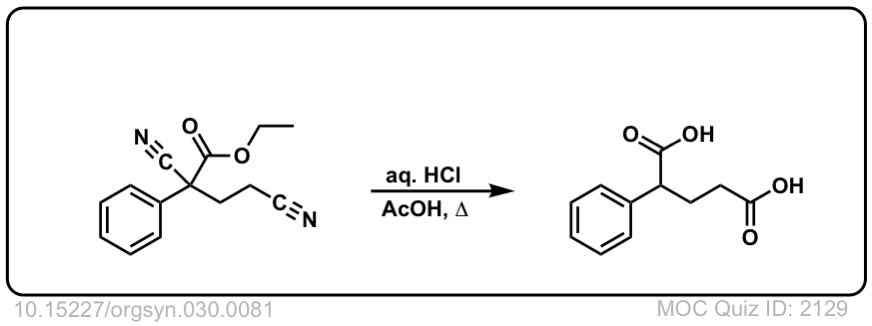
Org. Synth. 1950, 30, 81
DOI Link: 10.15227/orgsyn.030.0081
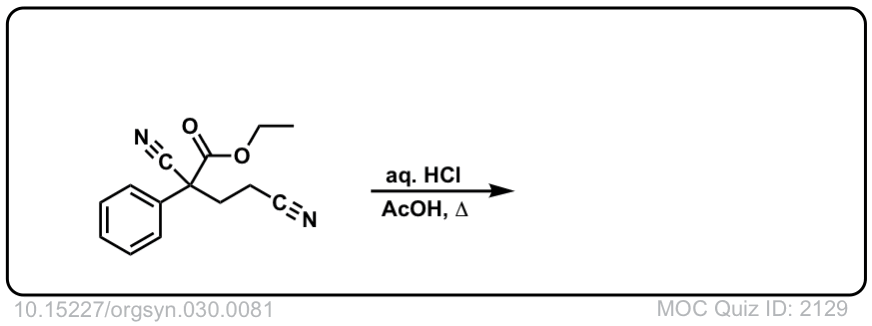 Click to Flip
Click to Flip
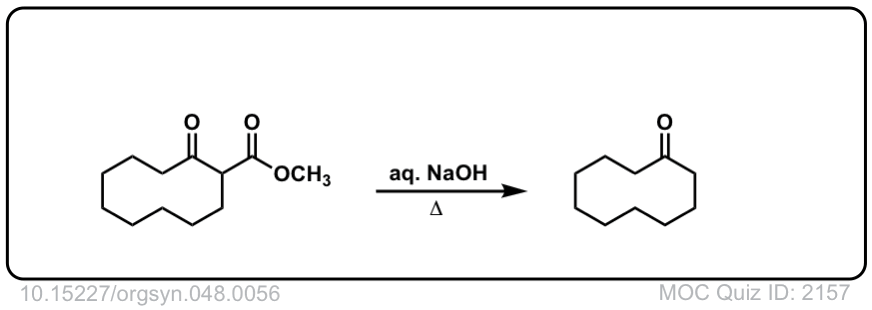
Org. Synth. 1968, 48, 56
DOI Link: 10.15227/orgsyn.048.0056
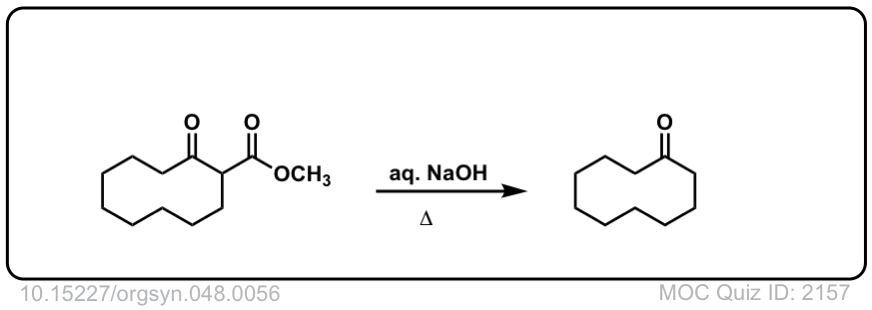 Click to Flip
Click to Flip
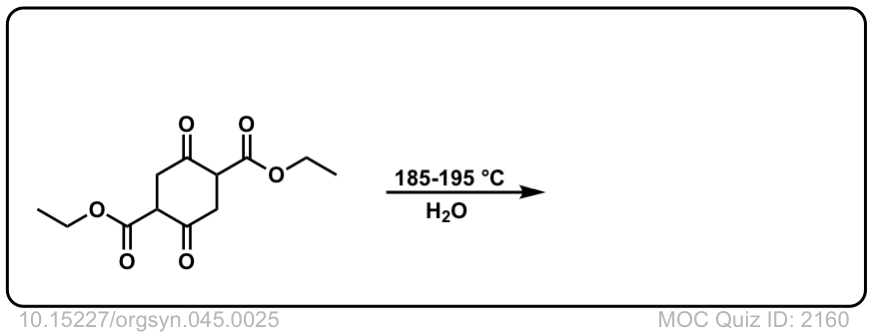
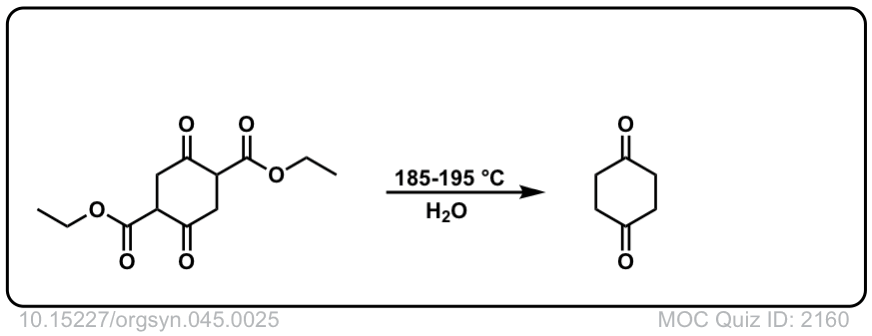
Org. Synth. 1965, 45, 25
DOI Link: 10.15227/orgsyn.045.0025
 Click to Flip
Click to Flip
We are doing carboxylation of Potassium 2,5-dichlorophenolate at 35 Bar and 120 deg C. But often we are getting very less conversion and are not able to understand the mechanism for the low conversion. IS the reaction irreversible and what are the parameters to control this
Yes, you’re forming a carbonate. You need to trap it somehow otherwise it will decarboxylate. But why are you doing this? Why not treat the phenolate with Boc2O or something and be done with it.
can you also explain the mechanism of heating of oxalic,succinic and adipic acid? plssss
Please can you explain sigmatropic reactions. I dont understand it
why beta keto acids are unstable?
Because they are likely to decarboxylate, as the title of this page suggests.