Electrocyclic Reactions
Description: Pi systems containing 2 or more conjugated pi bonds can undergo ring closure under thermal (heat) or photochemical (light) conditions. This is called electrocyclic ring closure. The reverse reaction (electrocyclic ring opening) is also possible.
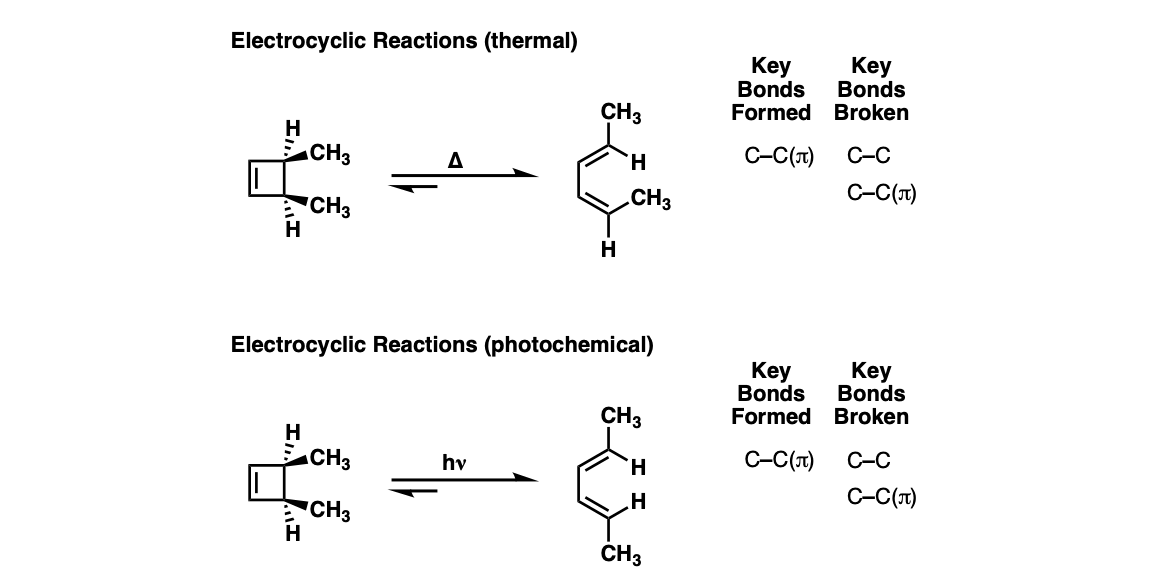
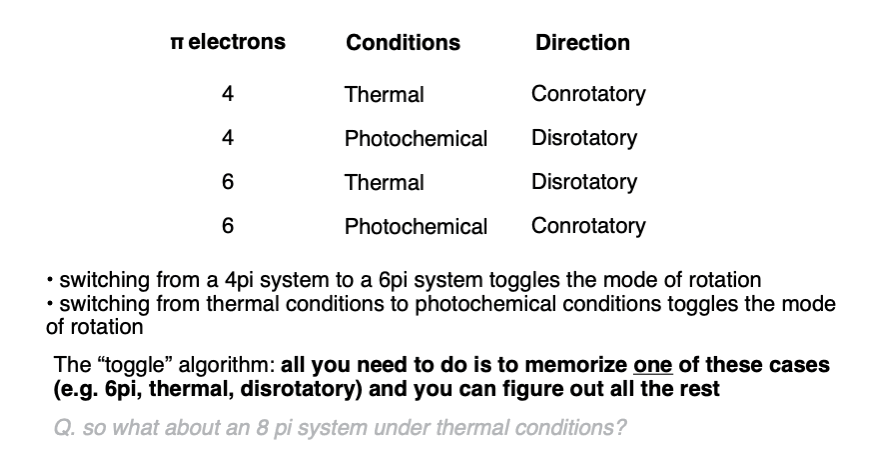
Notes: The stereochemistry of the ring opening/closure will depend on the number of pi electrons as well as whether heat ( Δ) or light (hν ) is used.
See these posts for more theoretical background on electrocyclic reactions.
- Electrocyclic Reactions (Part 1, butadiene and cyclobutene)
- Electrocyclic Reactions (Part 2, hexatriene)
Examples:
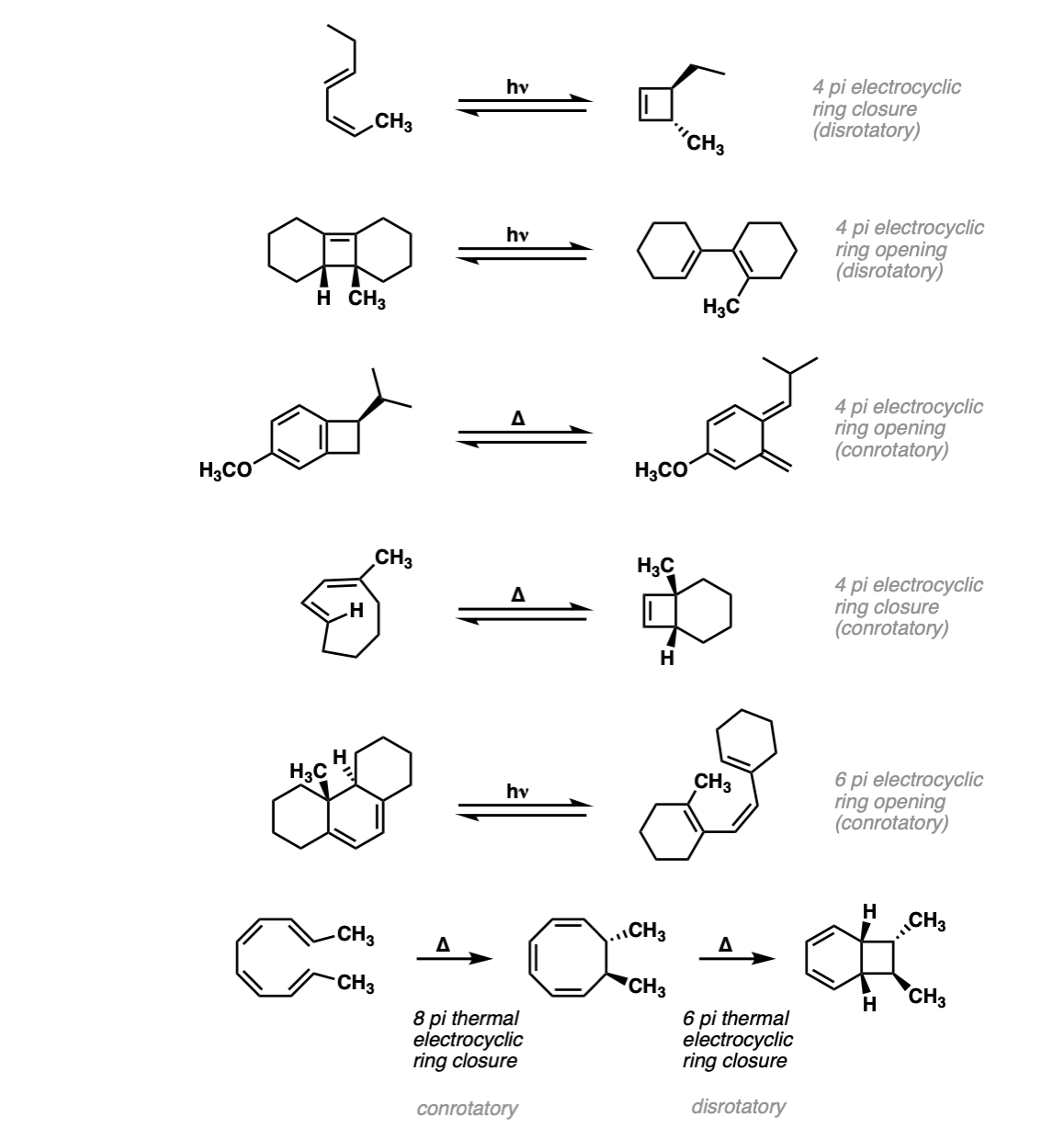
Notes: The first example is a 4pi electrocyclic ring closure under photochemical conditions. The second example is a 4pi electrocyclic ring opening. The third example is a 4 pi electrocyclic ring opening. (Note that this creates a new diene, which can be used for Diels-Alder reactions).
The fourth example is a thermal electrocyclic ring closure. The fifth example is a photochemical 6pi electrocyclic ring opening. The last example is two consecutive electrocyclic reactions – an 8pi thermal electrocyclic ring closure (conrotatory) followed by a 6 pi thermal electrocyclic ring closure (disrotatory).
Mechanism: Electrocyclic reactions proceed through a cyclic, concerted transition state.
In the first example below, the C1-C2 pi bond breaks at the same time the C3-C4 sigma bond breaks, with simultaneous formation of the C2-C3 and C1-C4 pi bonds (Step 1, arrows A and B).
In the third example below, the C1-C2, C5-C6, and C3-C4 pi bonds all break at the same time, forming the C1-C6 sigma bond, the C4-C5 pi bond, and the C2-C3 pi bond.
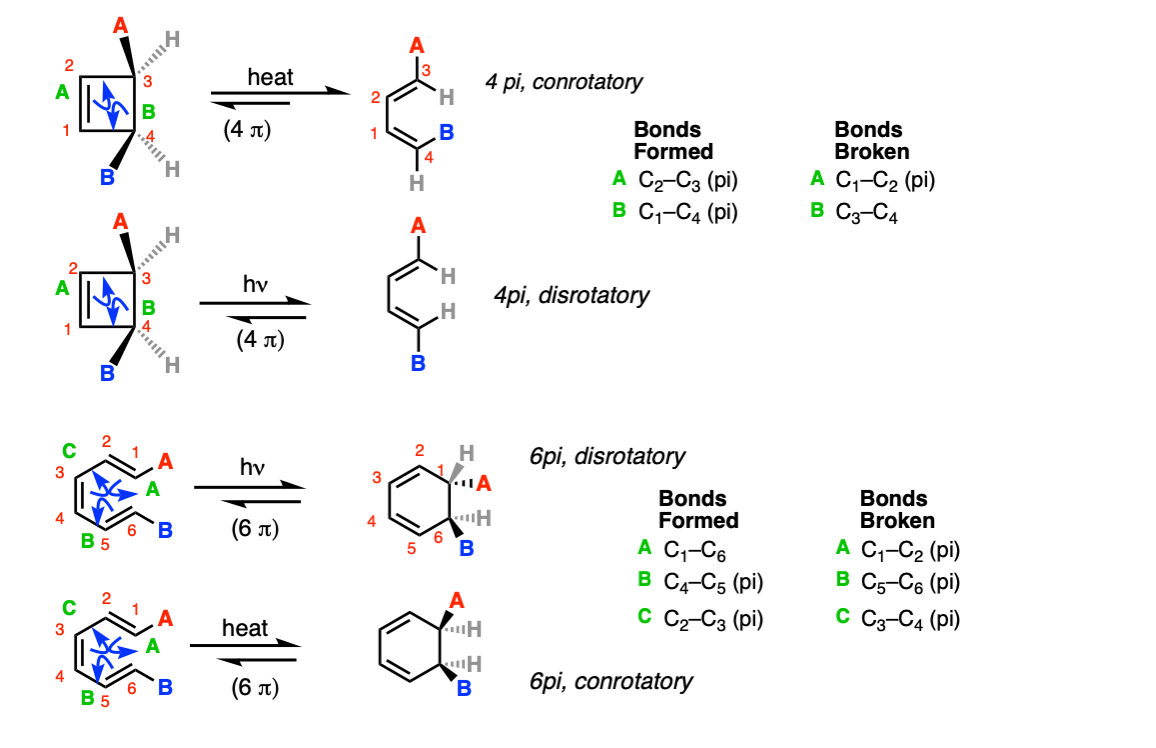
Notes: The mechanism for the ring opening reactions is the exact reverse of ring closure.
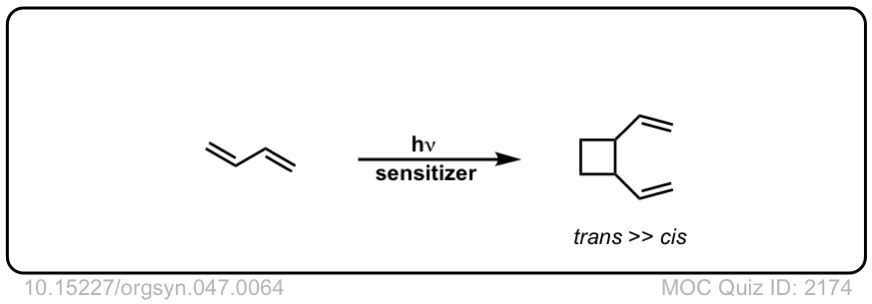
Real-Life Examples:
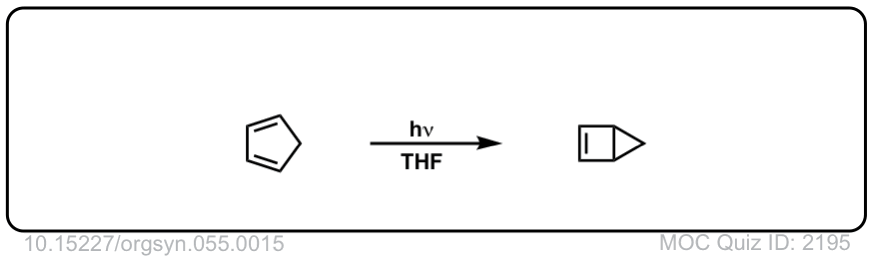
Org. Synth. 1967, 47, 64
DOI Link: 10.15227/orgsyn.047.0064
 Click to Flip
Click to Flip
Org. Synth. 1976, 55, 15
DOI Link: 10.15227/orgsyn.055.0015
 Click to Flip
Click to Flip