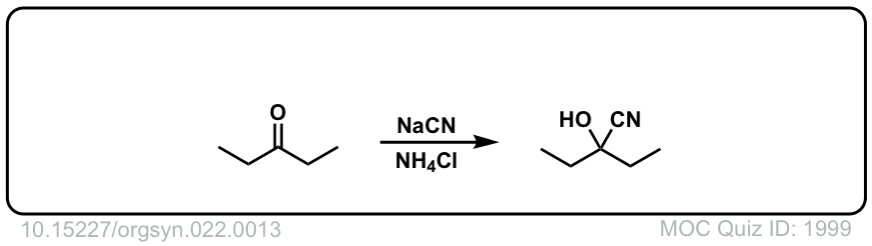
Formation of Cyanohydrins from ketones and aldehydes
Description: Treatment of aldehydes (or ketones) with cyanide ion in the presence of a proton source (acid) leads to formation of cyanohydrins.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
Org. Synth. 1942, 22, 13
DOI Link: 10.15227/orgsyn.022.0013
 Click to Flip
Click to Flip
Org. Synth. 1935, 15, 3
DOI : 10.15227/orgsyn.015.0001
Thank you. It’s a simpler explanation but far more effective than the one that I’ve found, which involved molecular orbitals and some work with Gaussian.
Hello James,
first of all congratulations on your hard work, it is really something. I’m here because I’m more interested in this material as a complement to teach. I’ve recommended to my students and I’m always looking for something fresh. Something like new applications for reactions, so on and so forth. Regarding this particular reaction I’ve found a question from a student (somewhere in the internet) inquiring why, regarding the cyanide ion, the nitrogen does not attack the carbonyl, since it has a pair of electrons on an sp orbital, and is far more electronegative than carbon. I believe the answer lies somewhere on atomic orbitals and its contribution to the molecular orbitals of CN. Could you share your thoughts? I really would appreciate.
Thanks,
Van.
If you look at the intermediate formed by attack of the nitrile lone pair, you’ll see that the nitrile nitrogen will become positively charged (and a great leaving group besides). It’s not a stable intermediate. While it’s possible that some of that intermediate will form, it will quickly revert back to starting materials.