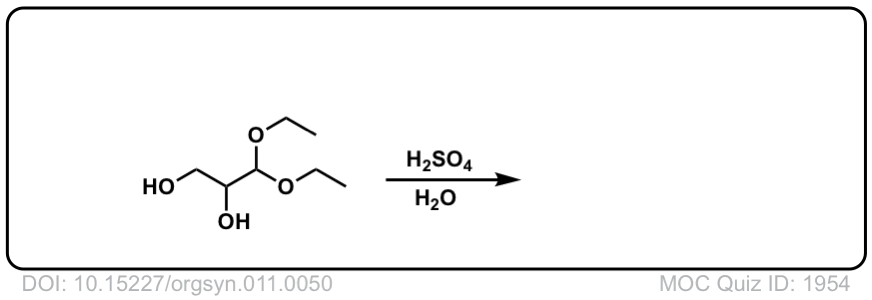
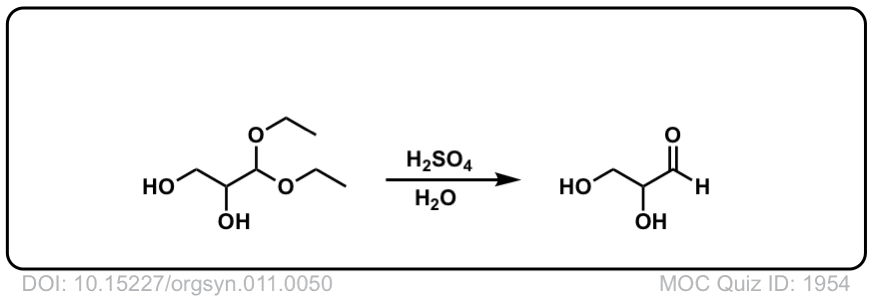
Hydrolysis of acetals to give aldehydes and ketones
Description: Addition of aqueous acid to acetals will transform them back into ketones (or aldehydes). This is often referred to as “deprotection” of ketones (or aldehydes).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1931, 11, 50
DOI Link: 10.15227/orgsyn.011.0050
 Click to Flip
Click to Flip
Can you explain the last bullet in the opening notes? Isn’t this deprotection and not protection?
The structure for TsOH is wrong. p-toluenesulphonic acid has a para methyl group….
cheers
Fixed. Thanks!
Hello – I am a bit confused about the introductory “notes” section. You state that the alcohol is used in great excess over water, but wouldn’t that lead to the formation of the acetal? I would imagine that having water in excess would drive the forward reaction instead.
you are right. Thanks for spotting! fixed the typo