Hydrolysis of imines to give ketones (or aldehydes)
Description: Treatment of imines with water leads to their hydrolysis back to aldehydes (or ketones) and an amine.
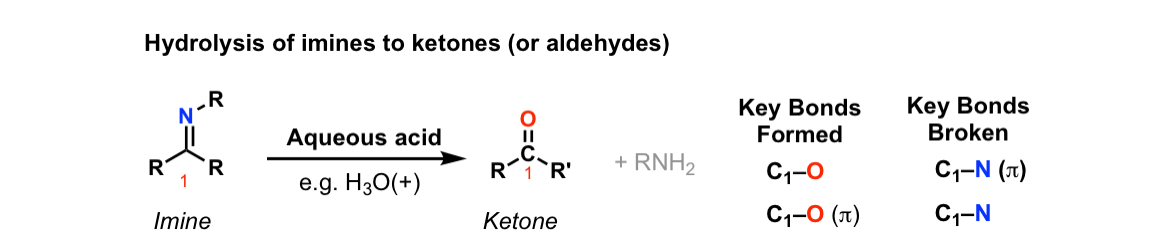
Notes: The reaction is assisted through the use of an acid catalyst.
Examples:
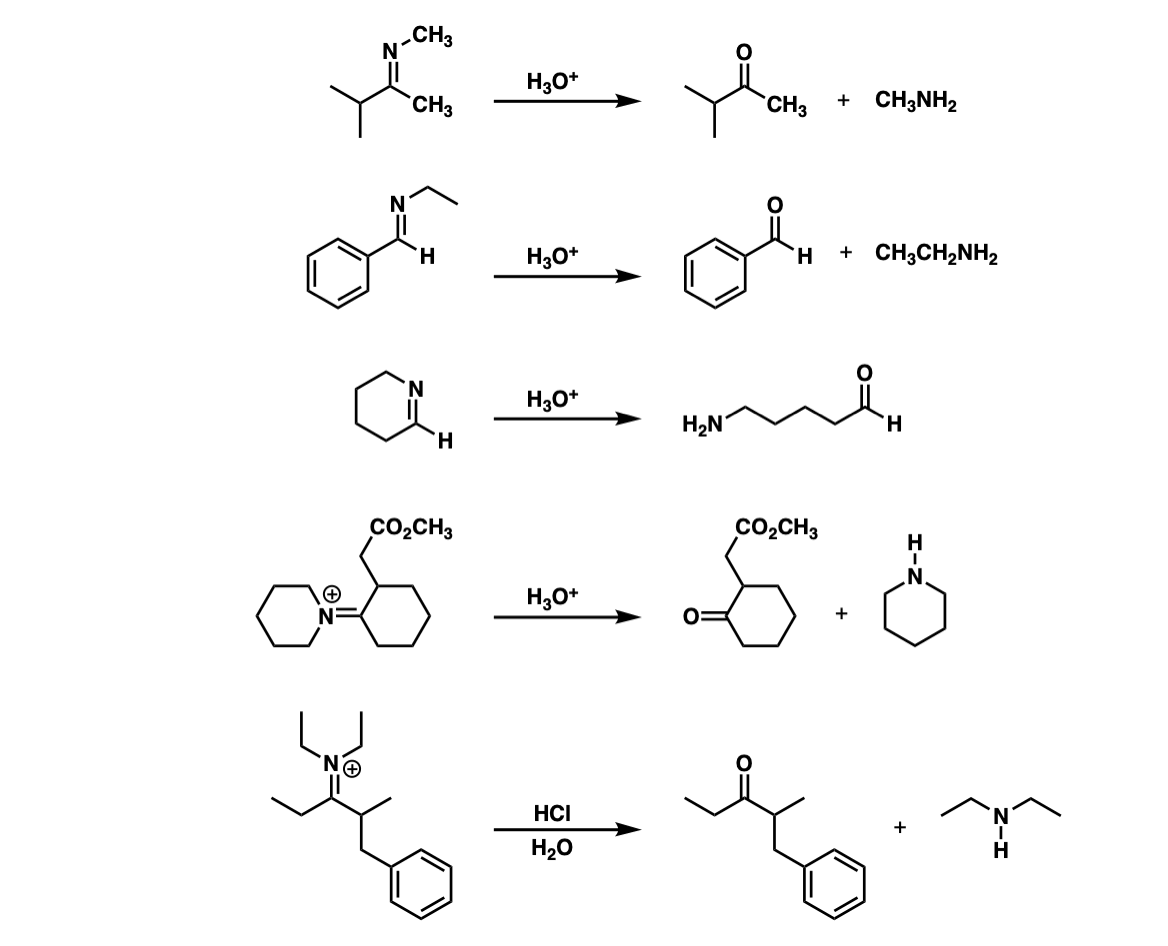
Notes: Note that the third example is intramolecular.
Mechanism: Protonation of the imine nitrogen (Step 1, arrows A and B) results in the formation of the iminium ion, which undergoes 1,2-addition by water (Step 2, arrows C and D). Transfer of a proton (Step 3, arrows E and F) followed by 1,2 elimination of ammonia (Step 4, arrows G and H) lead to an oxonium ion, which is then deprotonated to give the neutral ketone.
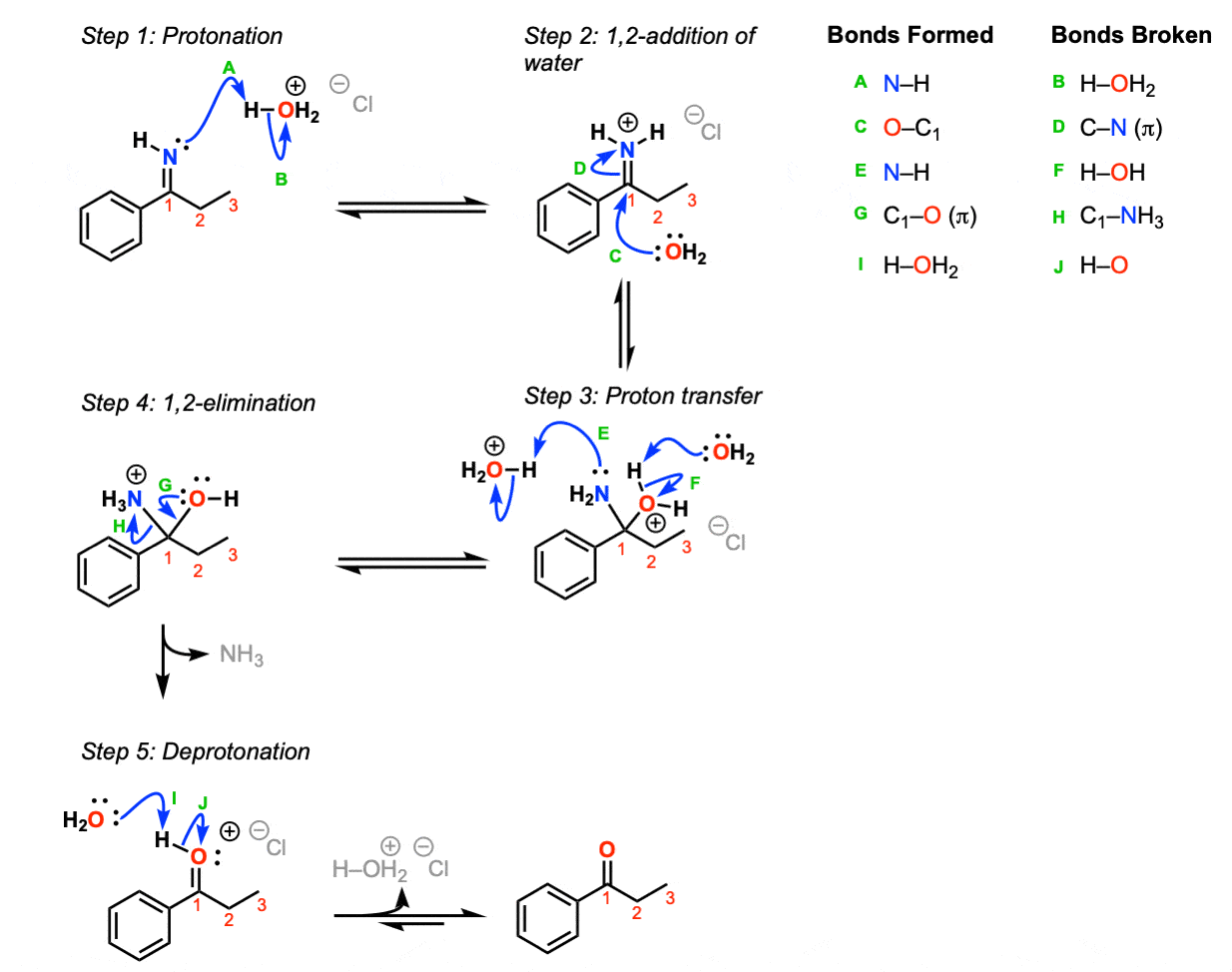
Notes:
- Acid is helpful but not an absolute requirement for this reaction. Reasonable mechanisms can be drawn without acid.
- The “Cl” here in H3O+ Cl- is completely unimportant, just meant to show a balance of charge for H3O+. Other counter ions such as Br-, HSO3-, etc. would work just as well.
- Note that this is an equilibrium reaction and goes in this direction because of the large excess of water. It is the exact reverse of imine formation.
- There are certainly other reasonable ways to draw proton transfer (Step 3) and other species besides H2O that could conceivably act as bases in the last step.
(Advanced) References and Further Reading
Imines are also known as “Schiff bases” in the classical organic chemistry literature, hence the name in the titles of some of these papers.
- On the Mechanism of Schiff Base Formation and Hydrolysis
E. H. Cordes and W. P. Jencks
Journal of the American Chemical Society 1962, 84 (5), 832-837
DOI: 10.1021/ja00864a031
In this classic paper, the authors present evidence that at neutral pH, loss of H2O is rate determining, but at acidic pH, attack of the amine is rate-determining. The maximum rate occurs near pH 4. - The Mechanism of Hydrolysis of Schiff Bases Derived from Aliphatic Amines
E. H. Cordes and W. P. Jencks
Journal of the American Chemical Society 1963, 85 (18), 2843-2848
DOI: 10.1021/ja00901a037
Under acidic conditions, the reaction proceeds through attack of water on the conjugate acid of the imine. In neutral and basic conditions, the rate-determining step is attack of water; under acidic conditions, the rate-determining step is decomposition of the tetrahedral addition intermediate. - Kinetics and mechanism of the hydrolysis of N-isobutylidenemethylamine in aqueous solution
Jack Hine, John C. Craig Jr., John G. Underwood II, and Francis A. Via
Journal of the American Chemical Society 1970, 92 (17), 5194-5199
DOI: 10.1021/ja00720a032
These papers provide experimental evidence for the varying mechanisms of the hydrolysis of imines in both acidic and basic media. - The Chemistry of Imines.
Robert W. Layer
Chemical Reviews 1963, 63 (5), 489-510
DOI: 10.1021/cr60225a003
Section IV.A. in this review (“Addition of Water”) has a short discussion on the hydrolysis of imines, which is a good place to get started. - Hydrolysis of imines: kinetics and mechanism of spontaneous acid-, base-, and metal ion-induced hydrolysis of N-salicylidene-2-aminothiazole
Anadi C. Dash, Bhaskar Dash, and Somnath Praharaj
Chem. Soc., Dalton Trans., 1981, 2063-2069
DOI: 10.1039/DT9810002063
Lewis-acid (e.g. metal ion) catalyzed hydrolysis of imines is also possible, as this paper describes. - Kinetic Study of the Hydrolysis of Schiff Bases Derived from 2-Aminothiophenol
Hassib, H.B., Abdel-Kader, N.S. & Issa, Y.M.
J. Solution Chem 41, 2036–2046 (2012)
DOI: 10.1007/s10953-012-9920-6
A very nice thorough investigation of the mechanism of hydrolysis of a specific imine, and includes the deriving of the specific rate equations for acid-catalyzed and base-catalyzed hydrolysis. - Deprotonation of the Schiff base of rhodopsin is obligate in the activation of the G protein
C. Longstaff, R. D. Calhoon, and R. R. Rando
PNAS June 1, 1986 83 (12) 4209-4213
DOI: 10.1073/pnas.83.12.4209
The chemistry of imines is of tremendous significance in biochemistry. Vision is based on the isomerization of retinal, which is bound to a protein called rhodopsin via a imine linkage through the amine side chain of a lysine
Hi,
Great explanation, thank you. I have a question about other similar reaktions. I was wondering if hydrazones and oximes go through hydrolysis as easy/in the same way since they, in my understanding, are basically the same reactions. Thank you.
Hydrolysis of oximes and hydrazones is not nearly as easy as hydrolysis of imines, but it does follow the same pathway. In practice more strongly acidic conditions are required and higher temperatures.
This is very helpful but I was wondering if since adding excess water pushes the reaction forward what would push the reaction backwards? Also if using acid as a catalyst can you add too much acid and if so what does that do?
Removing water would drive the reaction back towards formation of the imine.
As far as imine hydrolysis is concerned, adding too much acid isn’t so much of a concern so long as there is some non-protonated water present (H2O).
However in the opposite direction (formation of an imine from aldehydes/ketones and amines) there IS such a thing as too much acid – if the solution is too acidic, then all the amine gets protonated and can no longer serve as a nucleophile. The ideal pH is around 4 and the reaction starts to shut down around pH 1.
Wonderful explanation. Thank you!!
A practical point to make on this reaction is that while the acid is catalytic, often the amine product will neutralise the acid. This means you may need to ensure you have an excess of acid to ensure the reaction will complete. A student learnt this the hard way recently when their reaction didn’t fully hydrolyse a diimine, due to miscalculation of the amount of HCl required.
Yes. Thank you for clearing that up, Ben.
is imine hydrolysis possible with a secondary ammonium ion?
I’m not sure what you mean. Could you please attach a picture.
Hi, I am confused because what if the imine had an R group and not an H. Would it just be protonated twice? thank you
If you’re referring to the mechanism, it would be exactly the same except your leaving group would be RNH2 instead of NH3. Hope that answers your question!
Is there another base hydrolysis of Imine mechanism?
The nitrogen has to accept a proton at some point in order to become a good leaving group. It’s possible to use NaOH/H2O (i.e. mildly basic conditions) but still that relies on the solvent acting as a proton donor.
Imine hydrolysis is possible with base.. Which is better acid hydrolysis or base hydrolysis?
Which will allow nitrogen to be a better leaving group, acidic or basic conditions?
Is the imine or ketone more thermodynamically stable? It would be nice to know when I am pushing more or less uphill against the thermodynamically favored molecule.
In my lecture, I was given a reaction of 1,3-diketones with hydrazine to make pyrazoles (no acidic catalyst). Does this mean the excess of hydrazine is implied, or is it the aromaticity of the pyrazole driving the product in the absence of a catalyst?
To clarify on the follow up for anyone interested:
Imine vs Ketone thermodynamic stability uncertain but not particularly relevant, as reactions are controlled by excess anyways. In the formation of the imine, a drying agent or molecular sieve will remove water in the equilibrium as it builds up as side product.
The reaction I supplied is called the Knorr pyrazole synthesis, and actually my lecturer just forgot to add an acidic catalyst (more likely assumed I should know it would be there!) Imines formed from hydrazine substitution are hydrazones.
Very very clearcut and nice explanation with mechanism……….Loved it!!!!
Hi
Thanks for the nice illustration.
My query is after removal of water is the resultant imine air stable?
Air stable? Absolutely. However in the presence of water it will hydrolyze.
Hello,
I want to transfer carvone semicarbazone (an imine of carvone) back to carvone using this reaction. Which concentrations are suitable to do this? Is it reasonable to use 0,1 M HCl for example?
In other words: at wich pH has the reaction maxiumum efficiency?
Kind regards
Jeroen Vandervelde
I don’t quite get it. Isn’t H2O a better leaving group then NH3 or in other words, isn’t the latter a better nucleophile? Why does then reaction proceed towards the ketone? Just because water is in excess?
Thank you for the clarification in advance…
Yes – the reaction is in equilibrium, but the high concentration of H2O pushes the reaction toward the hydrolysis product.
Thank you for your fast reply. Let me just say that this site is exceptionally educational and helpful!
Cheers…
Imines are not stable it will undergo hydrolysis to get carbonyl compounds
hi,
is it possible to get imine from cyanide by making the use of grignard reagent?
Yes, but isolating the imine is not always very easy.
I’m not sure if this question belongs in this topic. But I’m just wondering is an iminium ion a stronger electrophile than an oxonium ion? It is in the Strecker synthesis, but I don’t exactly understand why.
Thanks this was really helpful!
Just one thing I’m still confused about… why can’t step 5 happen before step 4? Wouldn’t it be more favorable to deprotonate the oxygen first and then have the lone pair kick down and get rid of LG?
Sure… but what’s going to deprotonate it? : – )
This is done under slightly acidic conditions.
Compare O-H (pka about 15) with protonated carbonyl (pKa -2)
in 4 the N is electron deficient which facilitate its removal with lone pair on oxygen. it is not possible in 3
I would not say that the nitrogen is “electron deficient”, I would say that it’s been converted into a better leaving group (i.e. weaker base)
Great review, but I’m a little confused as to where the fifth carbon went on the intramolecular imine hydrolysis under examples?
It’s a typo. Fixed – thanks for spotting it!
Great outline! Excellent helping me right before my exam!