Keto-enol tautomerism
Description: Ketones and other carbonyl compounds containing a hydrogen adjacent to the carbonyl are in equilibrium with a constitutional isomer called an enol.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
Org. Synth. 1952, 32, 97
DOI Link: 10.15227/orgsyn.032.0097
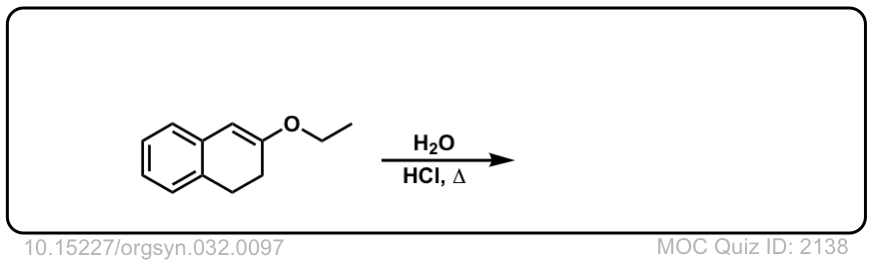 Click to Flip
Click to Flip
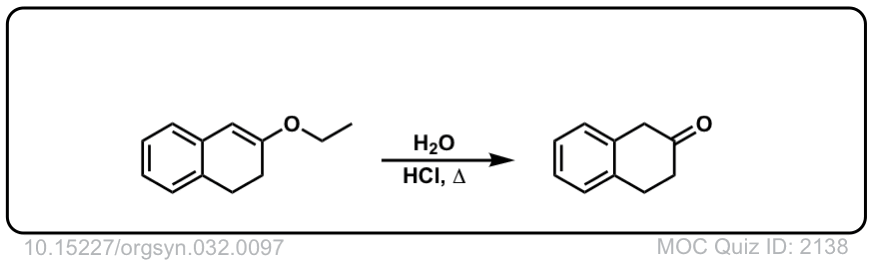
why when the oxonium ion come form enol the deprotonation for oxygenatom…??
and if it comes from keto form ( with acid) the deprotonation for alpha proton
in both cases is the same intermediate??
No video
The Video Walkthrough is down.
Thanks, I need to fix this.
which conditions are suitable for amide tautomerism …………..for proton abstraction
Hi! i read through your notes for keto-enol-tautomerism. I just have a questions, why is 2,4-pentandione is more favourable as an enol? is it because of the conjugation? could you please explain to me why? Thank you.
Two reasons! 1) if one ketone is present as an enol, then it can participate in a hydrogen bond with the other ketone carbonyl, which is a favorable interaction. And yes 2) it is also conjugated, so that has an impact as well.