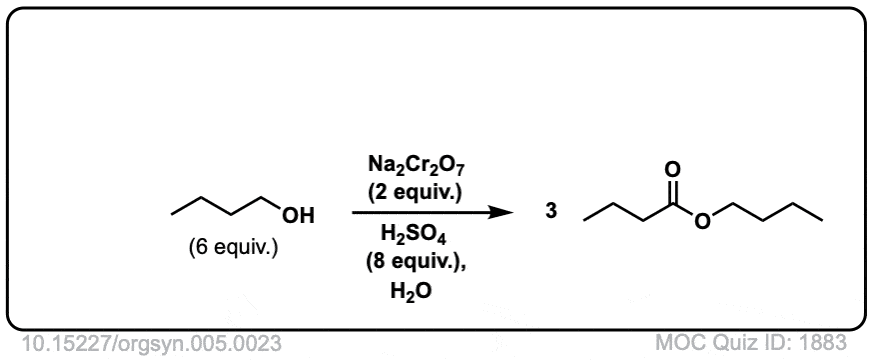
Oxidation of primary alcohols to carboxylic acids
Description: Primary alcohols treated with chromic acid will be converted to carboxylic acids.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1925, 5, 23
DOI Link: 10.15227/orgsyn.005.0023
 Click to Flip
Click to Flip
Hey James, I see that the H2O is still written as OH. Would it change the mechanism at all if this is changed to the correct form of H2O?
Not really, there would have to be an extra deprotonation step however.
Hello. This may be a silly question but why is H2O not shown as a part of the hydrate molecule shown in step 4 (i.e. it is only shown as OH)?
That’s not silly, that’s a good question. I’ll fix this.
its too nice website.i like it so much..