Oxidative cleavage of alkenes to give ketones/carboxylic acids using ozone (O3) – (“oxidative workup”)
Description: Ozone will cleave carbon-carbon double bonds to give ketones/carboxylic acids after oxidative workup.
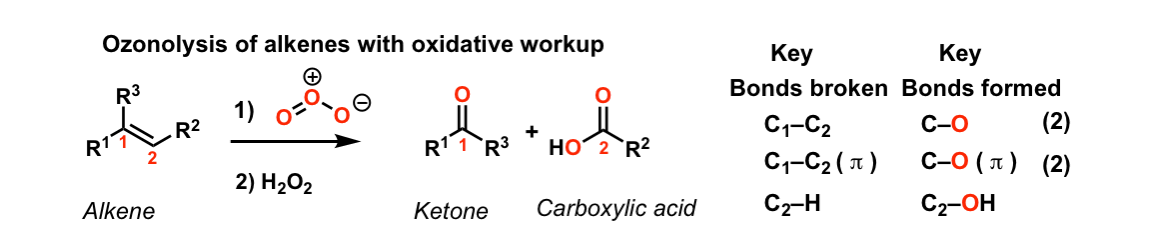
Notes: The initial product of this reaction is an ozonide. Treatment of the ozonide with acid and an oxidant such as hydrogen peroxide (H2O2) will convert any aldehydes to carboxylic acids. Note that any C–H bonds on the alkenes are converted to C–OH bonds, giving carboxylic acids.
Examples:
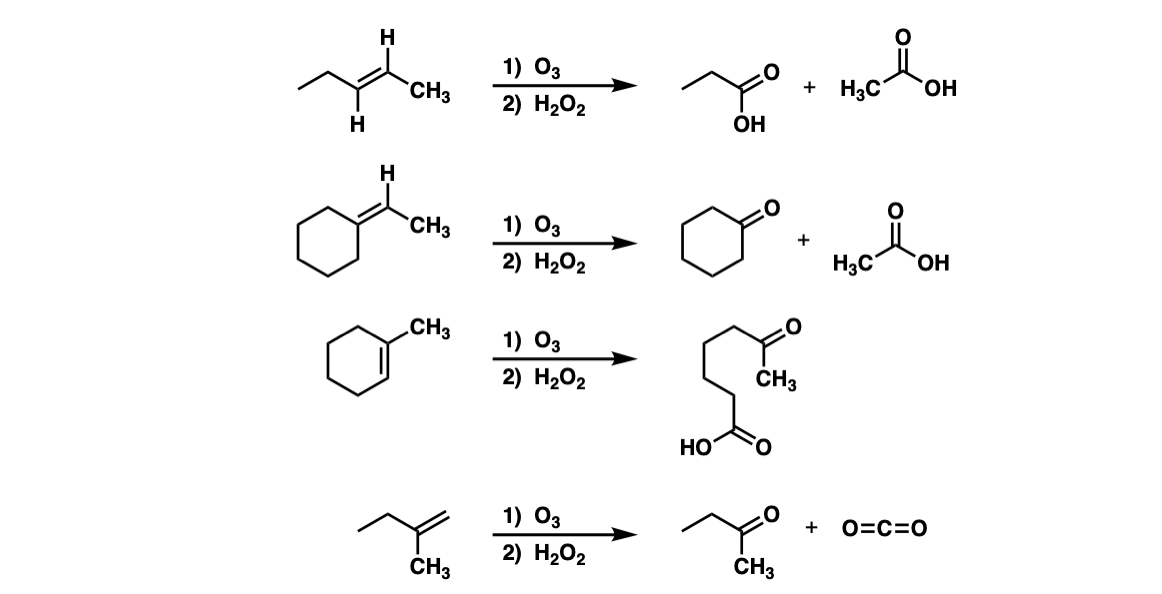
Notes: Note how every C–H bond on the alkene is converted into a C–OH bond to give a carboxylic acid. Also note that example 3 shows cleavage of a cyclic alkene to give a linear compound. In example 4, cleavage of a terminal alkene results in CO2.
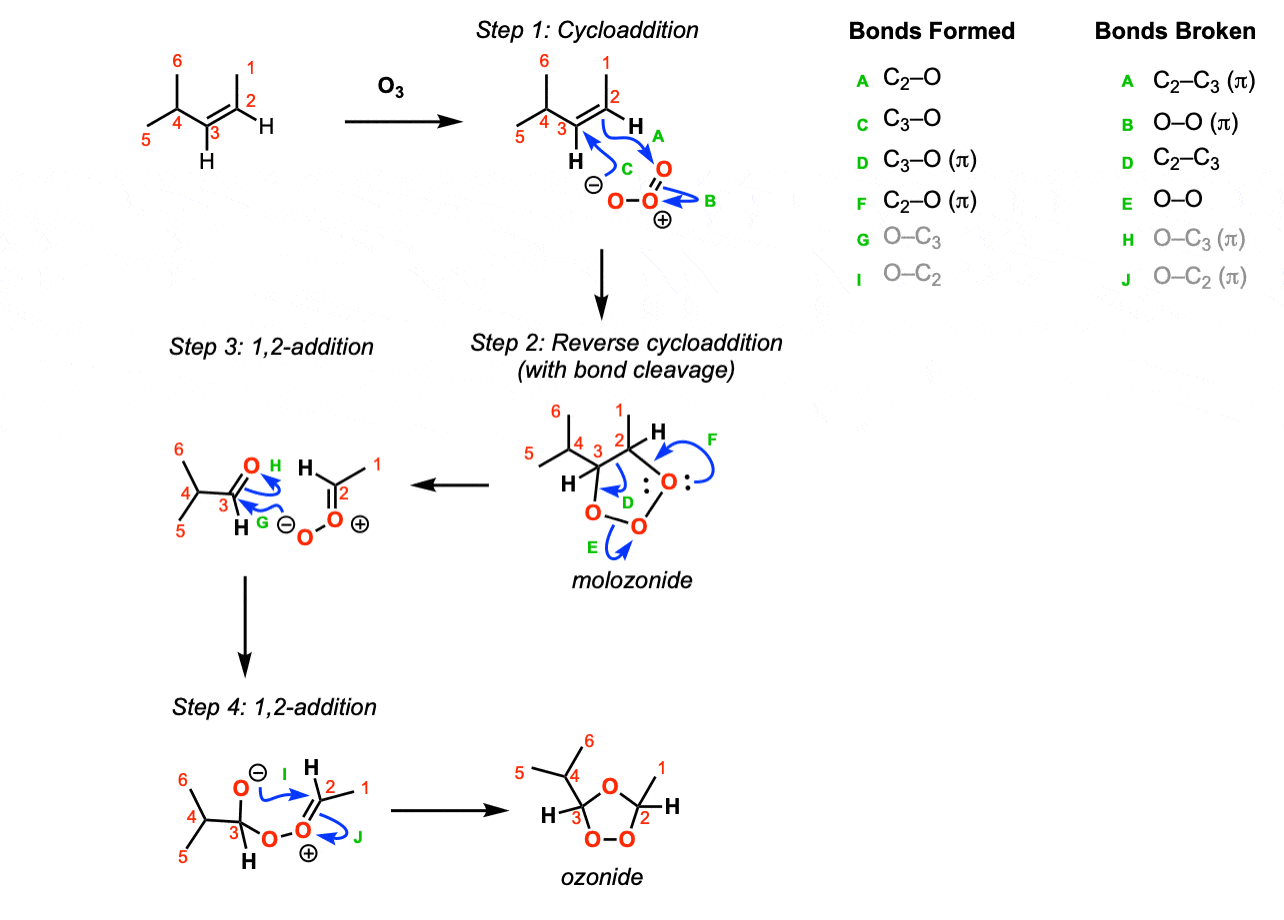
Mechanism: The first step of the reaction is a cycloaddition of ozone with the alkene (Step 1, arrows A, B, and C). The second step is a reverse cycloaddition, resulting in cleavage of the carbon-carbon single bond (Step 2, arrows D, E, and F). The oxygen of the carbonyl oxide then performs a 1,2-addition on the other carbonyl (Step 3, arrows G and H) giving a negatively charged oxygen that performs a 1,2-addition on the carbonyl carbon of the carbonyl oxide to give the ozonide (Step 4, arrows I and J).
With warming, the ozonide breaks down to the aldehyde and a carbonyl oxide (Step 5, arrows K, L, and M). Addition of peroxide to the aldehyde then occurs (Step 6, arrows N and O). This is followed by proton transfer (Step 7, arrows P and Q) and then removal of a proton with base to give the carbonyl (C=O) (Step 8, arrows R, S, and T).
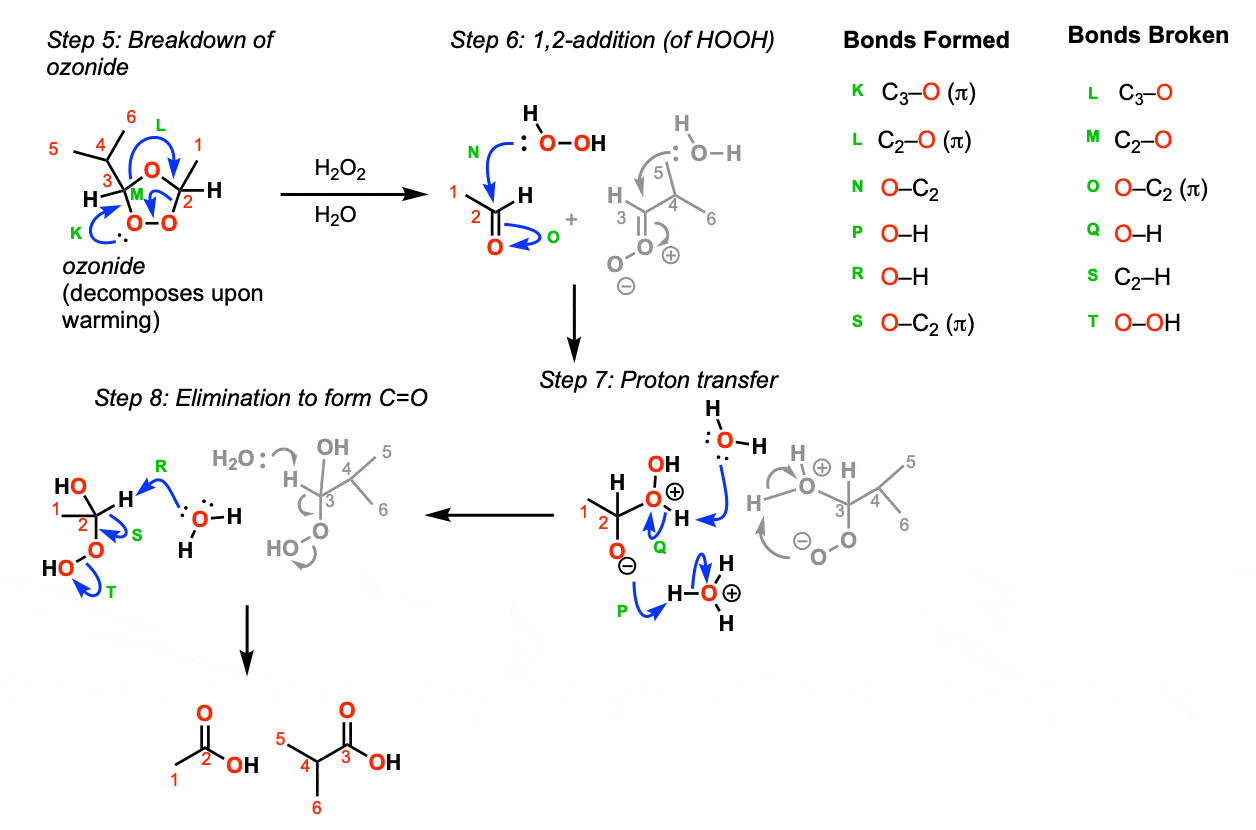
Notes: There are other reasonable ways to draw this mechanism, particularly other ways of drawing proton transfer in Step 7 and other species that could act as bases in Step 8.
(Advanced) References and Further Reading
blah blah blach placeholder blach
- Ueber die Einwirkung des Ozons auf organische Verbindungen
C. Harries
Just. Lieb. Ann. Chem. 1905, 343 (2-3), 311-344
DOI: 10.1002/jlac.19053430209
The first paper describing the oxidative cleavage of unsaturated compounds with ozone in solution. - OZONE
L. I. Smith, F. L. Greenwood, and O. Hudrlik
Org. Synth. 1946 26, 63
DOI: 10.15227/orgsyn.026.0063
This procedure from Organic Syntheses, a source of reliable, reproducible and independently tested organic chemistry laboratory experimental procedures, provides a detailed explanation of how to build a laboratory ozonizer. - The Preparation of Aldehydes, Ketones, and Acids by Ozone Oxidation
Albert L. Henne and Philip Hill
Journal of the American Chemical Society 1943 65 (5), 752-754
DOI: 10.1021/ja01245a003
This paper shows that carboxylic acids are formed in good yields from aldehydes when the ozonolysis reaction mixture is worked up in the presence of excess hydrogen peroxide. - Synthesis of sesquiterpene antitumor lactones. 7. Studies directed toward the total synthesis of pentalenolactone. Intramolecular ene reaction.
Frank Plavac, Clayton H. Heathcock
Tetrahedron Letters 1979, 20 (23), 2115-2118
DOI: 10.1016/S0040-4039(01)86277-8
This paper shows how ozonolysis with oxidative workup (7 -> 8) can be used in organic synthesis. - Ozonolysis of Cyclic Olefins
E. Franz, W. S. Knowles, and C. Osuch
The Journal of Organic Chemistry 1965, 30 (12), 4328-4330
DOI: 10.1021/jo01023a514
The transformation of VII to IX involves oxidative workup of the intermediate VIII using peracetic acid. One of the authors in this paper, W. S. Knowles, later received the Nobel Prize in Chemistry in 2001 for his work on asymmetric reduction reactions. - Ozonolysis of unsaturated phosphorus compounds
John K. Stille and J. L. Eichelberger
The Journal of Organic Chemistry 1971, 36 (13), 1840-1841
DOI: 10.1021/jo00812a029
This paper is by the same Prof. J. K. Stille of Stille reaction fame. As he says in the first paragraph, ozone is rarely used in organophosphorous chemistry, but here he demonstrates its utility in accessing phosphines that would be difficult to make by other means. - Oxidative cleavage of mono-, di-, and trisubstituted olefins to methyl esters through ozonolysis in methanolic sodium hydroxide
James A. Marshall and Albert W. Garofalo
The Journal of Organic Chemistry 1993, 58 (14), 3675-3680
DOI: 10.1021/jo00066a019
This paper describes workup conditions that directly yield esters. Using alcohols in the workup along with neutral or oxidative conditions will trap the resulting acids as carboxylic esters.
What if I use TME for ozonolysis? There’s no hydrogen for it to be replaced by OH, then what would be the products? I heard that it will be 2 ketones but I couldn’t figure out the mechanism…
Tetramethyl ethylene? This would give two equivalents of acetone (2-propanone).
Hello Sir. Is the any difference when we do oxidative ozonolysis in presence of H2O and other in presence of H2O2?
H2O2 will oxidize any aldehydes to carboxylic acids. H2O will not play any role other than as a solvent.
Hello Mr. Ashenhurst. I see you say that the oxidative workup always gives carboxylic acids, BUT the second picture, first reaction, has 2-pentene going through the oxidative workup and the products shown are an aldehyde and an acid. Was that a mistake? If not, why does that happen?
That is a mistake. I will fix it right now. Thank you for the correction!
Thanks so much James, really fabulous website..
in second example, is there enantiomers?
No, there are no chiral carbons present, so no enantiomers.
James, Does cleavage of a terminal alkene always result in CO2? A different example I found elsewhere of an alkene using reagents O3 and H2o2 on a terminal alkene gave 1 carboxylic acid and 1 aldehyde. Is this possible?
I found similar information on other sources, and am slightly confused too!! ^^^^ Could you clarify the rules for terminal alkenes with oxidative and reductive workups with ozonolysis, as well as with oxidative cleavage with kmno4 and heat?
Hi Nikki – for ozonolysis, the key difference between oxidative workup and reductive workup is this:
reductive workup leaves any C-H bonds alone
oxidative workup (incl. KMnO4) oxidizes any C-H bonds on the double bond to C-OH
So with a terminal alkene (like 1-butene): for a reductive workup the products will be a three-carbon aldehyde and a one-carbon aldehyde (formaldehyde).
for oxidative workup the products will be a three carbon carboxylic acid and a one carbon acid (O=C(OH)2 “carbonic acid”.) Carbonic acid will lose water to give carbon dioxide.
Has ozone in this mechanism got anything to do with 1,3 dipoles? If so, does it mean that this resonance struture with dipoles on opposite ends of ozone is significant?
Yes – ozone is a “1,3 dipole” meaning that – as you mentioned – the resonance form with negatively charged oxygen on one side and positively charged oxygen on the other is very significant. There is a large class of reactions known as “1,3 dipolar cycloadditions” of which the reaction of ozone with alkenes is just a single example.
The very last image doesn’t seem to be working for me!
fixed. Thanks!
Thanks James. What about cobalt and other transition metals? Is there a good resource to read more about the reactions of water/oxygen/peroxides/carbonyls with alcohols/olefins? Thanks in advance.
Is it possible to react organic peroxides with alkenes to get carbonyl groups? Only H2O and O2 would be present. What about primary alcohols?
By themselves, peroxides will not lead to cleavage of alkenes. Peroxides can lead to oxidation of aldehydes to carboxylic acids, but by themselves, will not oxidize alcohols to aldehydes. There are metal-based catalysts capable of doing both transformations with peroxide as a reoxidant, however.
Nice article! Thanks.
Is it be possible to cleave the alkene only by hydrogen peroxide, as ozone have safety problems commercially.
No, never. You need a strong oxidant like KMnO4 or O3.
Oh! I mean is there a reagent/mechanism that can revert the 6-oxoheptanoic acid back to the methylcyclohexene?
The closest mechanism I got to was:
1. reduce the 6-oxoheptanoic acid with LAH
2. protect the alcohol that formed where the carboxylic acid was with dihydropyran
3. oxidize the ketone-turned alcohol back in a ketone
4. treat with acid to retrieve the carboxylic-formed alcohol…. but from here, I get an ester if i use the carboxylic-formed alcohol to attack the ketone..
For example 3, is there a way to go back to the original 1-methyl-1-cyclohexene??
I can’t seem to get the cyclic structure back…
I’m not sure what you mean by “go back” to the original. If you mean, understand how the product came from the starting material, I’d suggest numbering all the carbons and going from there.