Oxymercuration of Alkenes to form Ethers using Hg(OAc)2
Description: When an alkene is treated with an alcohol in the presence of mercuric acetate [Hg(OAc)2] it will add to the more substituted position of the alkene (Markovnikoff) to give an ether. The mercury can then be removed using NaBH4.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
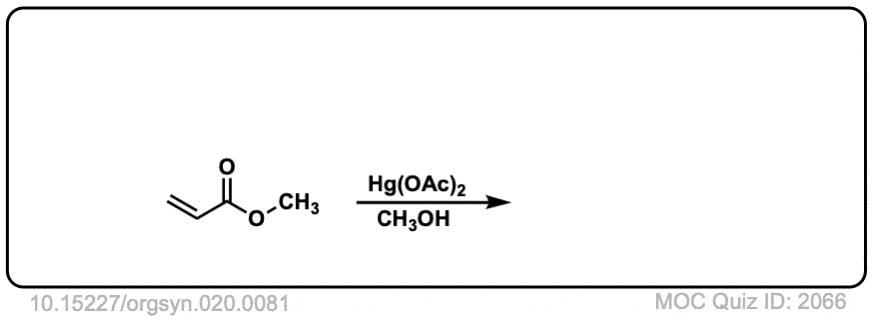
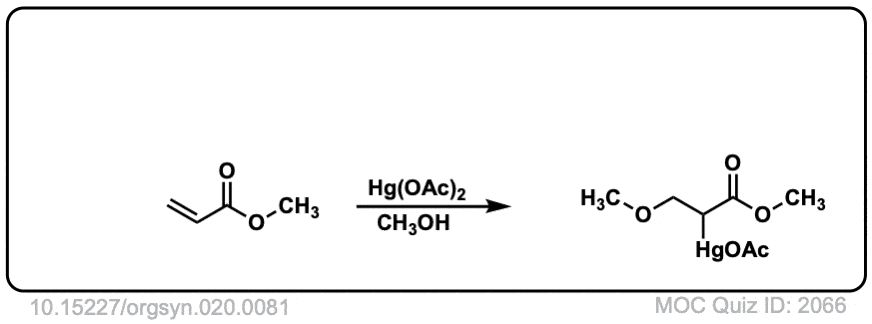
Real-Life Example:
Org. Synth. 1940, 20, 81
DOI Link: 10.15227/orgsyn.020.0081
 Click to Flip
Click to Flip
I think I realized a mistake in the Reactions of Alkenes Summary Sheet. Stereoselectivity of Oxymercuation-demercuration reaction is given as both syn+anti, however it should be anti (addition of H and OH on opposite faces) addition which gives the major product.
Hi – the addition itself is anti.
However, when the C-Hg bond is cleaved, it results in a free radical on carbon, and the free radical can react with H on either face. The result is that the anti stereoselectivity is lost and it becomes a mixture of syn and anti.
Why is the real-life example anti-markovnikov?
Great question!
It’s adjacent to an electron-withdrawing ketone. Normally you would expect the more substituted carbon to be the best at stabilizing positive charge, but the electron-withdrawing influence of the ketone means that it is in fact the less substituted carbon that is better at stabilizing positive charge.
Similar “anti-Markovnikov” selectivity is observed in addition to alkenes where one side has a CF3 group or other electron-withdrawing groups.
why is the double bond positioned on the outside of the ring (for step 1)?
I wouldn’t say that it’s positioned on the outside of the ring. It’s an attempt to show the double bond of the ring forming a three-membered ring with mercury.
For the removal of mercuric acetate why must sodium borohydride be added in a basic condition?
The title says “to form alcohols” but isn’t that “ether” instead?
Fixed. Thank you!
In the last example why wasn’t ROH used along with Hg(OAc)2
the OH end of the alkene is the ROH in this case
That is absolutely correct Taylor. Thanks.
okay lang. add more example.