Wohl Degradation
Description:
The Wohl Degradation is a very old-school method for reducing the length of a sugar by a single carbon, like the Ruff degradation. Yields are… not great.
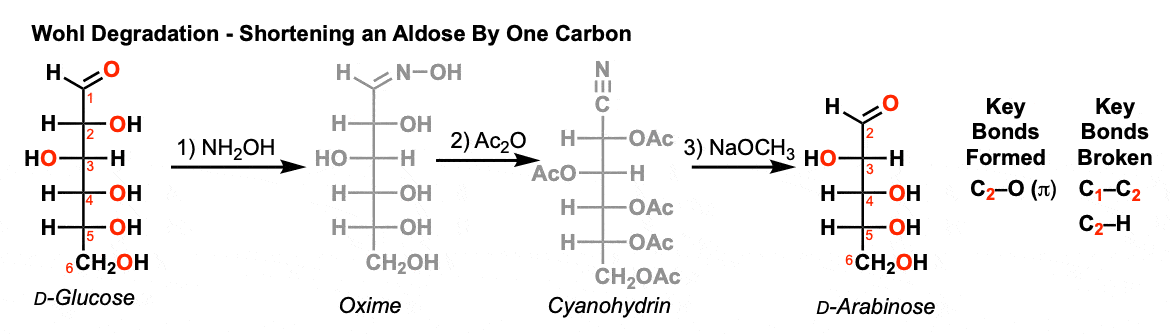
Comments: The Wohl degradation is composed of 4 individual reactions.
- In the first reaction, an aldehyde is converted into an oxime through the addition of hydroxylamine (NH2OH)
- In the second step, all the oxygens are converted into esters with an acylating reagent such as acetic anhydride (Ac2O). The purpose here is to make the oxygen attached to nitrogen a better leaving group.
- The third reaction is a Beckmann rearrangement where the C-H bond migrates to the nitrogen, breaking the N-O bond. After deprotonation, the result is a nitrile.
- These three reactions result in a cyanohydrin.
- In the last step, treatment of the cyanohydrin with base results in loss of (-)CN (a reasonably good leaving group) to give the chain-shortened sugar.
Examples:
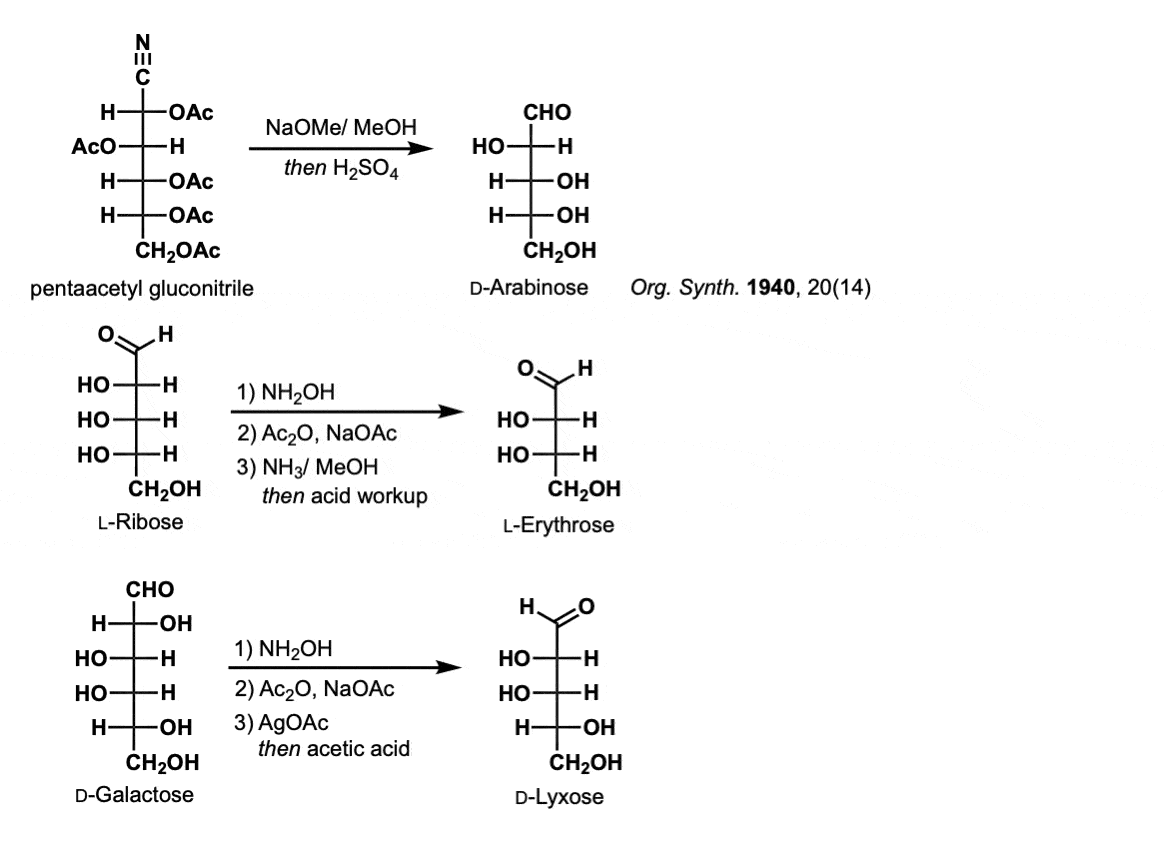
Comments: The first example shows treatment of the cyanohydrin with base to give the chain-shortened aldose (D-Arabinose). Addition of base also results in cleavage of the acetate groups. The second example shows the complete process of the Wohl going from L-Ribose to L-Erythrose. The third example shows formation of D-Lyxose from D-Galactose. Note that the choice of base (NaOCH3, NH3, AgOAc) is not important for our purposes.
Mechanism:
The Wohl degradation is built up of three different processes. Let’s walk through oxime formation first.
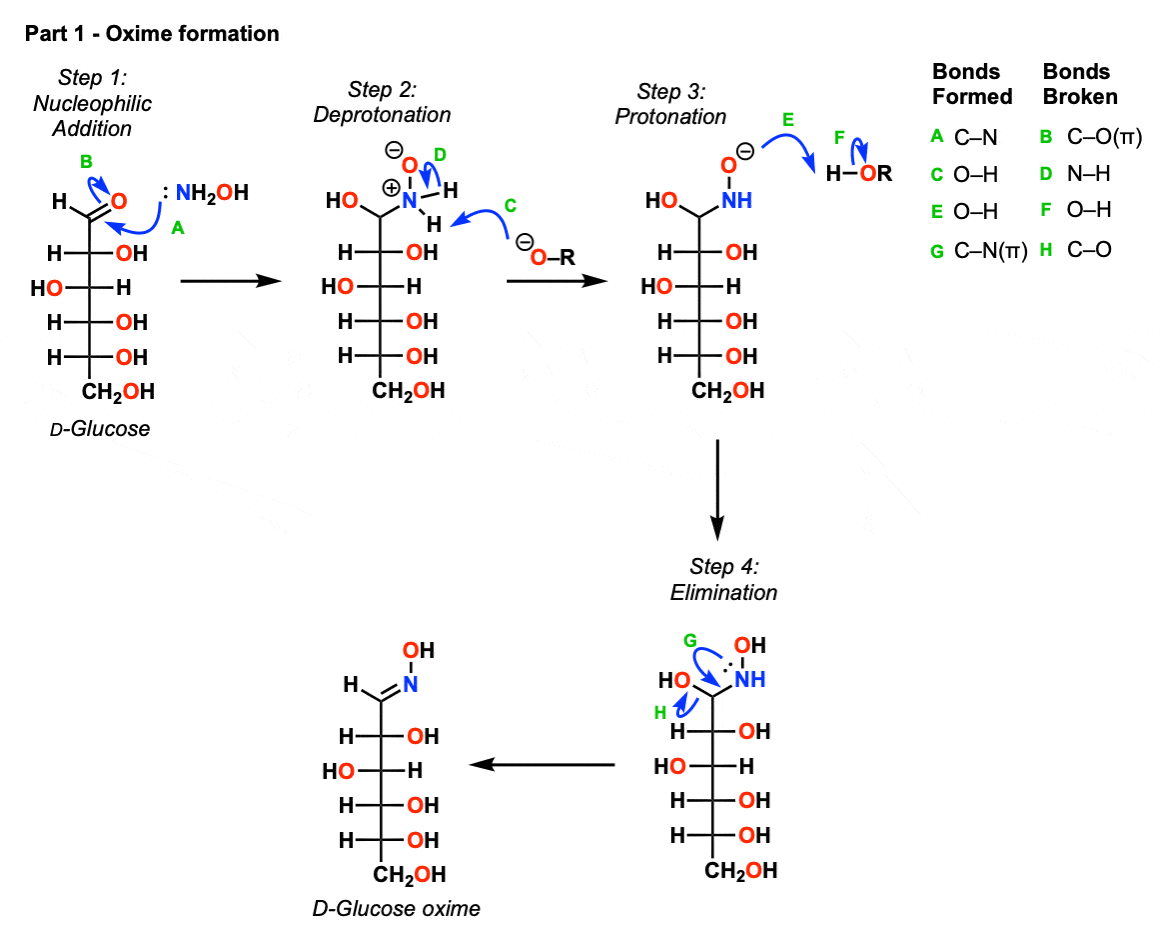
Part 1 – Oxime formation
Here, hydroxylamine (NH2OH) performs an addition reaction on the aldehyde carbonyl of the aldehyde (Step 1, arrows A and B) resulting in formation of a tetrahedral intermediate. This then undergoes proton transfer in two steps, shown here with deprotonation by base (Step 2, arrows C and D) followed by protonation at oxygen (Step 3, arrows E and F) to give a neutral tetrahedral intermediate (a “hemiaminal”) which then undergoes elimination of hydroxyl ion (Step 4, arrows G and H) to give the neutral oxime.
So 1) addition, 2) proton transfer, followed by 3) elimination.
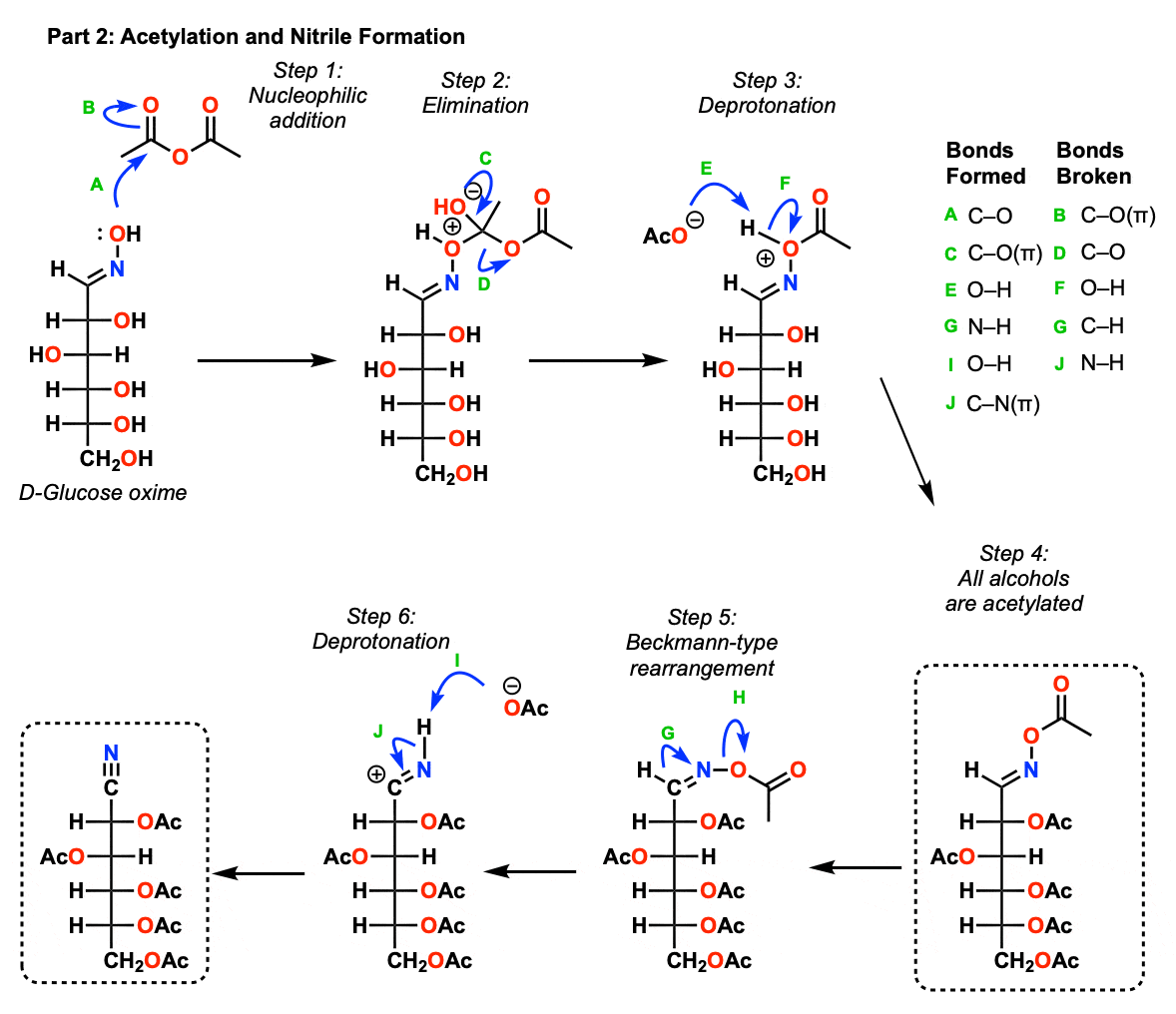
Part 2: Acetylation and Nitrile Formation
In the next step, we add acetic anhydride (Ac2O) which is going to put acetate groups (Ac) on all of all oxygens including the oxygen of the oxime.
This occurs through addition of the oxime oxygen to the carbonyl of acetic anhydride (Step 1, arrows A and B) followed by elimination of acetate ion (Step 2, arrows C and D) and subsequent deprotonation of oxygen (Step 3, arrows E and F). All alcohols are also acetylated through a similar process.
The next step is a little weird. This is is essentially a Beckmann rearrangement The N-O bond is very weak. Heating results in a 1,2-shift of hydride from the oxime carbon to nitrogen, where the N-O bond breaks -think of it like a backside attack (Step 5, arrows G and H). A lone pair from nitrogen forms a new C-N pi bond giving the nitrile, which is deprotonated at nitrogen. (Step 6, arrows I and J).
[Advanced note – the N-O bond was drawn differently in step 5 in order t0 show this hydride shift onto the backside of the nitrogen, where there is an empty N-O antibonding orbital]
This finally results in an acetylated cyanohydrin which is going to undergo elimination in the next step.
Part 3- Aldehyde formation
In the grand finale we treat the acetylated cyanohydrin with base and this will end up losing nitrile anion to give the aldehyde.
In the first step, methoxide ion performs an addition reaction on the carbonyl (Step 1, arrows A and B) followed by elimination to give an alkoxide anion (Step 2, arrows C and D). All the other acetate groups are removed at this time.
Since cyanide (-CN) is a good leaving group, an elimination can then occur where a new C-O (pi) bond forms with loss of cyanide (Step 3, arrows E and F). This gives us our final chain-shortened product.
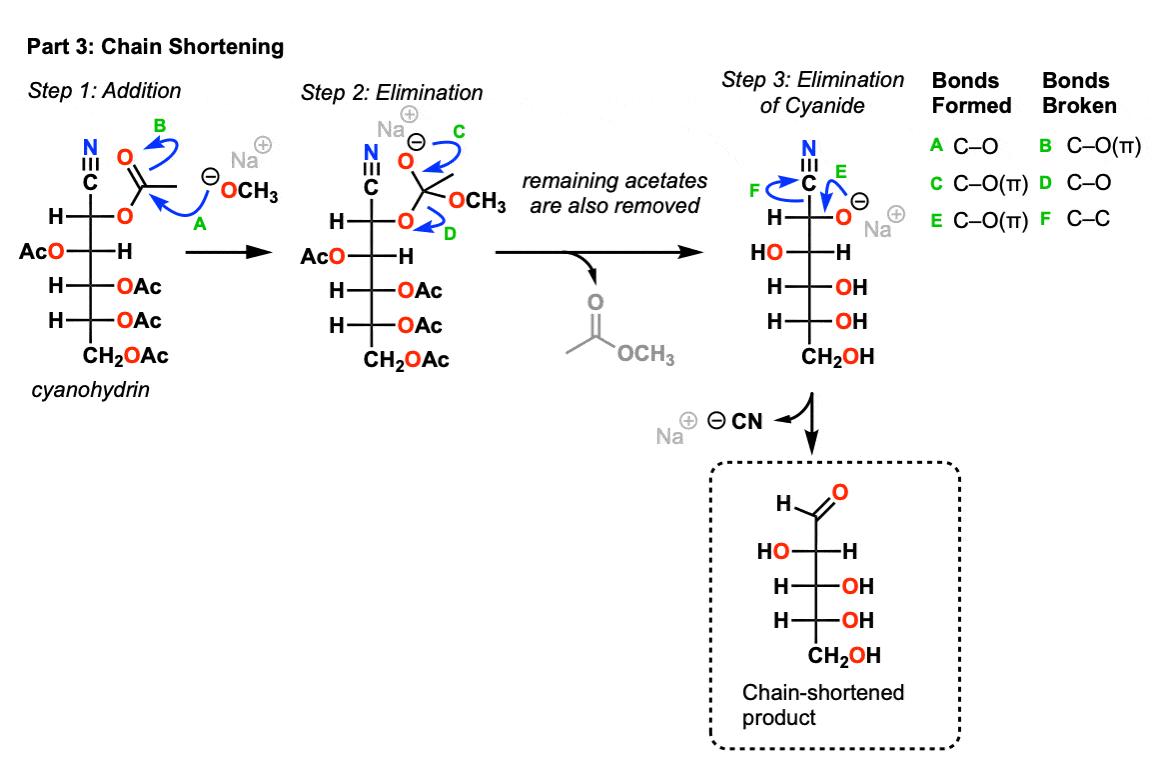
Notes: Other bases can be used for this reaction as well besides methoxide. Some use ammonia; silver acetate has also been used.
(Advanced) References and Further Reading
- Abbau des Traubenzuckers
Wohl, A.
Chem. Ber., 26 (1): 730–744
DOI: 10.1002/cber.189302601150
The original report of the chain shortening of sugars that bears Wohl’s name. - D-ARABINOSE
Braun, G.Org. Synth. 1940, 20, 14DOI: 10.15227/orgsyn.020.0014
This article shows a detailed, reproducible procedure for effecting the formation of D-arabinose from glucose (penta-acetyl D-gluconitrile). - PENTAACETYL d-GLUCONONITRILE
H. T. Clarke and S. M. Nagy.Org. Synth. 1940, 20, 74This article has a detailed preparation for the formation of pentaacetyl D-gluconitrile from D-glucose. When combined with the previous reference, these two preparations show a complete Wohl degradation.