Diels Alder Reaction of dienes and dienophiles
Description: The Diels Alder reaction converts a diene and an alkene (usually electron-poor, called a “dienophile”) into a six-membered ring containing an alkene (cyclohexene).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
Org. Synth. 1952, 32, 41
DOI Link: 10.15227/orgsyn.032.0041
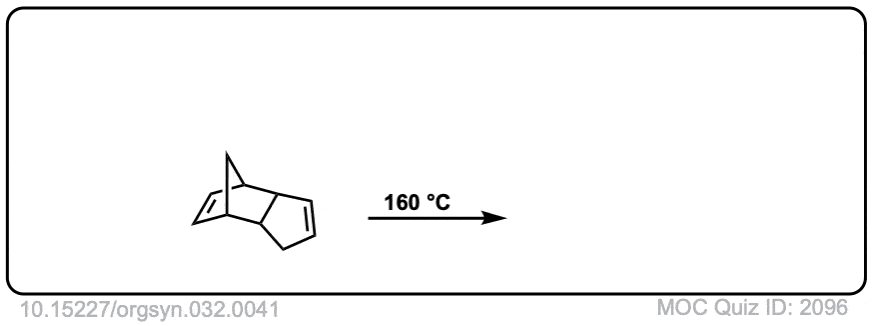 Click to Flip
Click to Flip
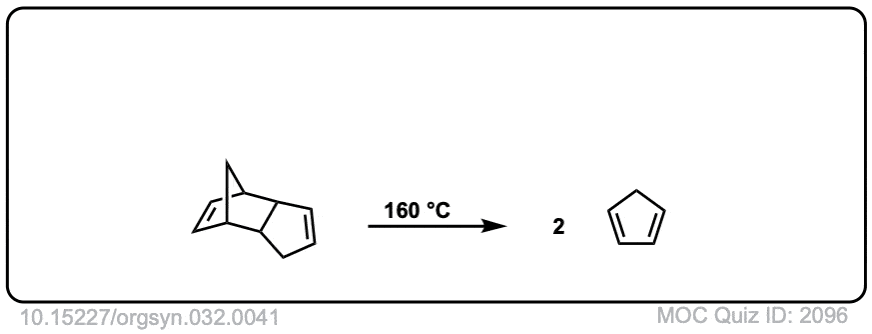
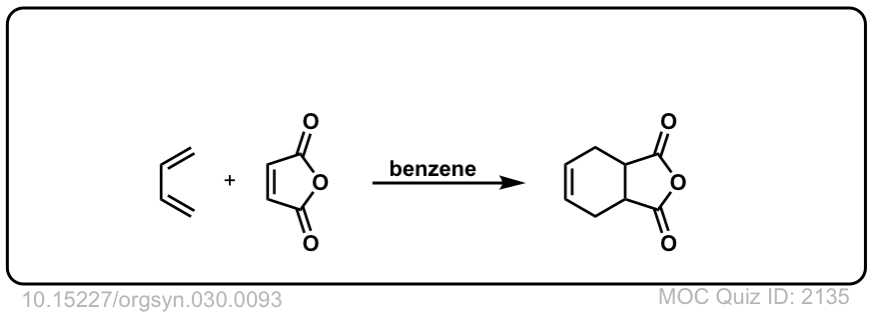
Org. Synth. 1950, 30, 93
DOI Link: 10.15227/orgsyn.030.0093
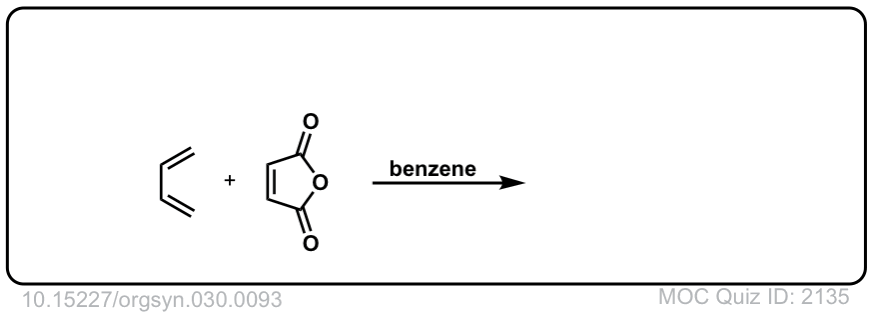 Click to Flip
Click to Flip
Org. Synth. 1969, 49, 1
DOI Link: 10.15227/orgsyn.049.0001
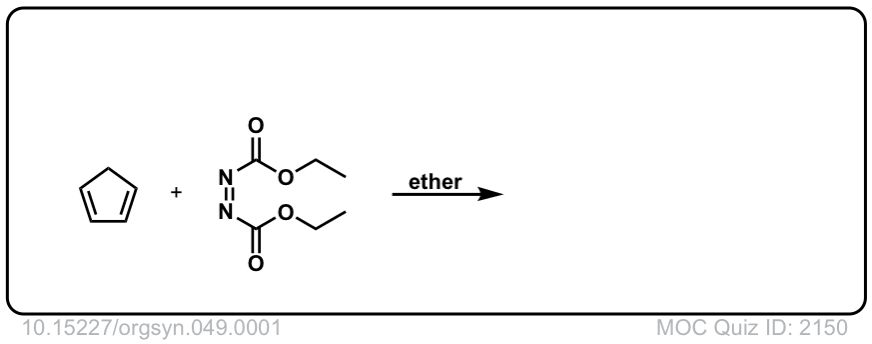 Click to Flip
Click to Flip
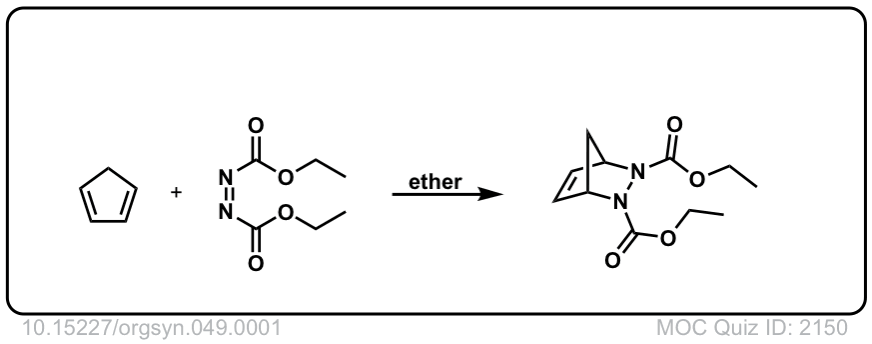
Org. Synth. 1962, 42, 50
DOI Link: 10.15227/orgsyn.042.0050
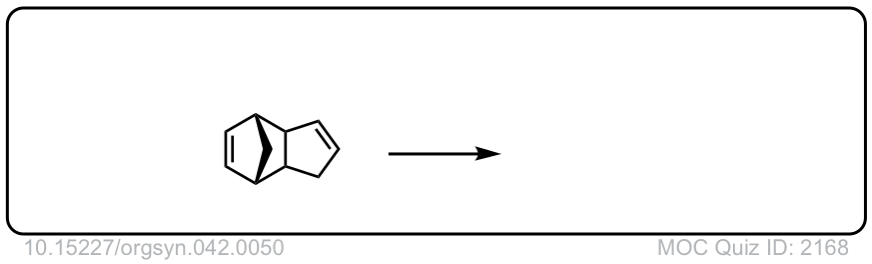 Click to Flip
Click to Flip
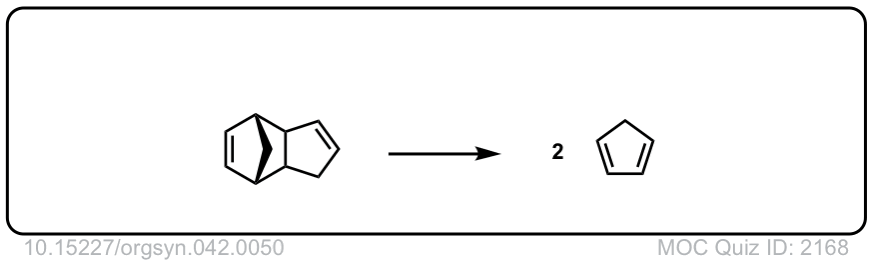
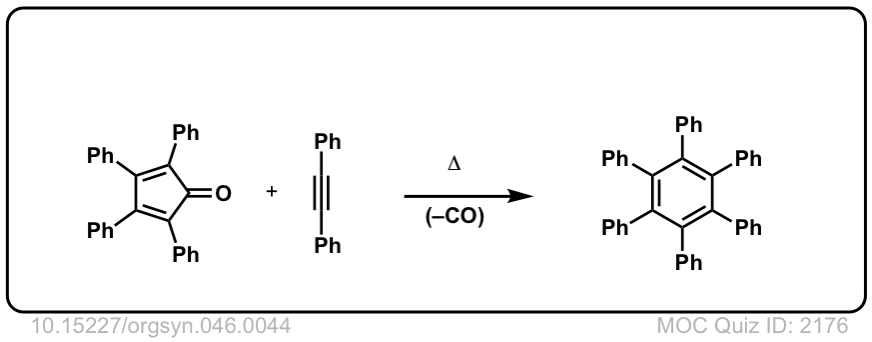
Org. Synth. 1966, 46, 44
DOI Link: 10.15227/orgsyn.046.0044
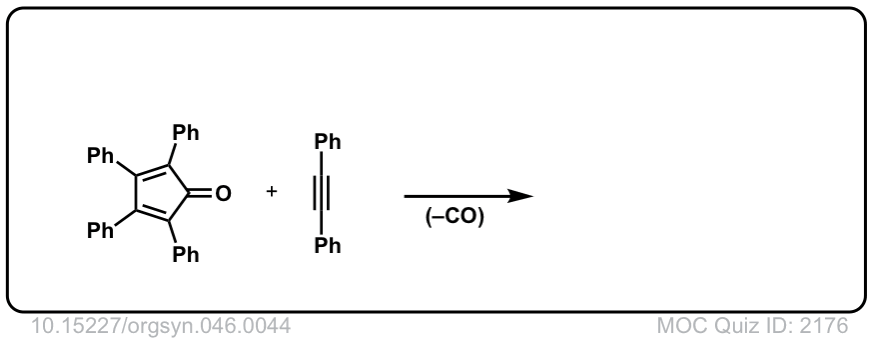 Click to Flip
Click to Flip
I’m really confused that for quiz ID:1712(the last one), why the product doesn’t have 2 pi-bonds as the quiz ID:1711 ?
Thank you so much.
I have got a question in Paula Bruice which as follows
1,3-Butadiene + Acetylene Cyanide = ?
The question in under the Diel-Alder synthesis part so I guess it is a similar reaction.
The place where I got confused is whether the C≡C will break or
C≡N.
Can you explain the major product and its reason?
For our purposes, Diels-Alder reactions will always break three C-C pi bonds and form two C-C sigma bonds and one C-C pi bond.
The CN will not participate.
I want to know why 2+2 cycloaddition is favored by photochemical process?
Read this post: https://www.masterorganicchemistry.com/2018/03/23/molecular-orbitals-in-the-diels-alder-reaction/
Dear James
Thanks a lot for your amazing website. Actually, I am working on a lecture in advanced organic chemistry for MChem. I just want to show my students how to use simple disconnections which lead to simple starting materials. Do you have examples for retrosynthesis of pericyclic stereoisomeric compounds in a way which leads to simple starting materials like aldehyde or ketone (Wittig … etc)?
Thanks a lot in advance
Best regards
Basil
Hi Basil – glad you find it useful. I’m not sure how to answer your question but I might suggest Chemistry By Design, which goes through various total syntheses step by step. https://chemistrybydesign.oia.arizona.edu/
My test is 3 hours away and I just started this…. I am diene
Good luck on your test!
I don’t understand how the last two reactions occurred. The only difference I noticed was the position of the CH3O group. Even though the difference was in the diene, the dienophile was the one that connected differently. For the last one (example 9), I would think that the ketone would still be facing down and C4 would be connected to C5 not C6.
I think you need to look at it again. Draw the most important charged resonance forms of the dienes and dienophile and line up the negative charge on the diene with the positive charge on the dienophile.
For compound 6, how would you determine whether this product has an enantiomer based on R and S configuration? I always use R and S to determine whether the compound is meso, so since the diels alder has compounds that are symetrical, with more than 2 chiral centers, how would you determine the configuration? I could tell if there were just two chiral centers but with alot of R and S going on in the molecule it’s a little difficult.
Thanks,
Sunidhi
The product is chiral – it has 3 chiral centers and no plane of symmetry. Therefore it will have an enantiomer. You don’t even need to get into R and S , but assigning R and S is helpful for determining whether compounds are enantiomers, diastereomers or the same. See https://www.masterorganicchemistry.com/2019/03/08/enantiomers-diastereomers-or-the-same-1-using-models/
Taking my OChem 2 Final tomorrow morning. Just want to say thanks. This blog got me through so much of the last two semesters, and I anticipated revisiting for the MCAT. I’ve referred this site to many many people. Thank you.
Thank you so much for the kind comment.
I have to ask if the alkyne react with a diene in diels Alder reaction which one will be the nucleophile and electrophile?
Looking forward for your kind support.
It depends on which is electron-rich and which is electron poor. But assuming an electron-rich diene like butadiene, one could use an electron-poor alkyne such as dimethyl acetylene dicarboxylate (DMAD) as the dienophile
Dear James,
Concerning example 8: Since oxygen is electronegative, shouldn’t the CH3O group on the diene be an electron-withdrawing group, not electron-donating? I am a bit confused.
Oxygen is a pi-donor, so if you draw the resonance form where the oxygen forms a C-O pi bond with carbon, that will push the C-C pi bond to give a negative charge on the end carbon. It actually makes the diene electron-rich!
Although you mentioned how the cycloaddition reaction can have the arrow pushing mechanism go clockwise or counterclockwise, in example 1, wouldn’t carbon 5 of the alkene be considered a better “electrophile” versus carbon 6? Therefore, wouldn’t it be more favorable to have the diene attack carbon 5 instead of carbon 6?
Thanks so much!
Actually carbon 6 would be a better electrophile – if you draw the “second best” resonance form you’ll see that it puts a carbocation on C-6 and since it is an important resonance contributor there will be partial positive charge on this carbon. See https://www.masterorganicchemistry.com/2018/11/05/regiochemistry-in-the-diels-alder-reaction/
Can anyone say the reactivity of following dienes in dielsalder reaction?
1.simple diene 2.cyclic diene 3. 1,3cyclohexene 4.simple diene with one of the πbond on other side 5.transoid form of diene(cyclic structure).
Too many variables to answer.
please explain examples 8 and 9
See this post: https://www.masterorganicchemistry.com/2018/11/05/regiochemistry-in-the-diels-alder-reaction/
Why the 2,5 and 1,5 arrangement of the products in reaction 8 and 9?
That’s a good question. I quote from my textbook: “We can predict the major product of unsymmetrical Diels-Alder reactions simply by remembering that the electron-donating groups of the diene and the electron-withdrawing groups of the dienophile usually bear either a 1,2-relationship or a 1,4-relationship in the products, but not a 1,3-relationship.” Can’t seem to figure out why. I think that’s just the way things work.
I have no idea when this comment is from, but it’s the day before my final, and this was incredibly helpful. Thank you so much!!
8.9 resonance can explain that
Is it possible to get a Diels Alder product with two double bonds present in the ring? One of my hw problems requires me to determine the diene and dienophile that would be required to synthesize an 8 carbon bicyclocompound that has two double bonds present opposite of each other. There is a methyl group and an aldehyde group flanking off of one of the double bonds. I know how to figure it out if there was just one double bond present in the Diels Alder product, but this second double bond is really throwing me off!
I think I would have to use an alkyne as my dienophile? I’m just confused because I thought the dienophile had to be an alkene. Also, just to clarify, the compound has a 6 carbon ring and a 2 carbon bridge.
Yes, it is possible to get a Diels-Alder product with two double bonds opposite each other in the ring. You use an alkyne instead of an alkene. You do the reaction just as you would with an alkene — don’t break or move the second pi-bond of the alkyne. Use one of the pi-bonds of the alkyne to form the new sigma bond between the diene and the dienophile, and the other pi bond stays there and becomes the second double bond.
You are a lifesaver brother. Thanks a lot!
Can you please explain in detail examples 8 and 9
I think another great example would be to add an intramolecular diene as well.
Hi,
Thank you for the illustration. Also, Lewis acid catalysis or increase of pressure will favor Endo product.
Hi,
In example 5, the product seems to be a meso compound and would not be (+ enantiomer), is this right?
Hi, example 5 is chiral because of the presence of the double bond. If the double bond were removed it would be achiral, but not meso, because if you try to determine R/S you will find that it’s attached to two identical groups!
Is there specific conditions in which the bridge , like the one formed in the second example, is formed?
Hi – a bridge would be formed in the Diels Alder when you have a cyclic diene, like cyclopentadiene or cyclohexadiene. Hope this helps – James
I have a similar reaction as in Example 3, where the diene is a conjugated fatty acid and the dienophile is fumaric acid.
That means the expected product would have two carboxylic acid groups on the dienophile.
Question: Is there a way to cleave that dienophile bond (between C5 and C6) after the product is formed?
I would think it would give my acid groups less steric hindrance to further react with other esters.
Let me know Thanks!
You wouldn’t be able to cleave it directly, because it’s a C-C single bond. In order to answer the rest of your question, I’d need more information about your specific system.
Wow this is very, very good… I was checking out the “endo and Exo” and though what is dienes and dienophiles – – and surprise I could get to this page right away and this is simply awesome…. thank you so much – when I check the formulas in Biology I always has some questions about this …. now answered THANK YOU !!!!
In regards to exo v. endo.- I was under the impression that while the exo is stericlly favored, the endo is favored as the major product due to secondary stabilization provided by overlap of pi bonds during the transition state.
Second, are the arrows actually only able to be represented as clockwise since the diene is symmetrical(final example), therefore having equal partial negative charges on each end from which electron density will be dispersed to the partial positive region on the dienophile? If the diene were not symmetrical, arrows would need to be from the partial negative regions to the partial positive regions, correct?
Note: Pertaining to example 6, the endo product is kinetically favored, while the exo product is thermodynamically favored.
It is a little tricky to explain, but basically the orbitals in the endo product line up well, so when there is not a lot of energy in the system, the reaction will go toward the endo product which has a lower transition energy. However, when there is more energy in the system the product will go toward the exo product because it is a lower energy state overall. You can see that the exo product would be more stable because there are less steric interactions.
Thank you for clarifying that point!
If I’m not mistaken, Clayden in his book outlines this very nicely with diagrams.
Extremely articulate. Thank you!
very very helpful…thanks