Formation of imines from primary amines and ketones
Description: Reaction of a primary amine with an aldehyde or ketone results in an imine. The reaction results in the formation of one equivalent of water.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
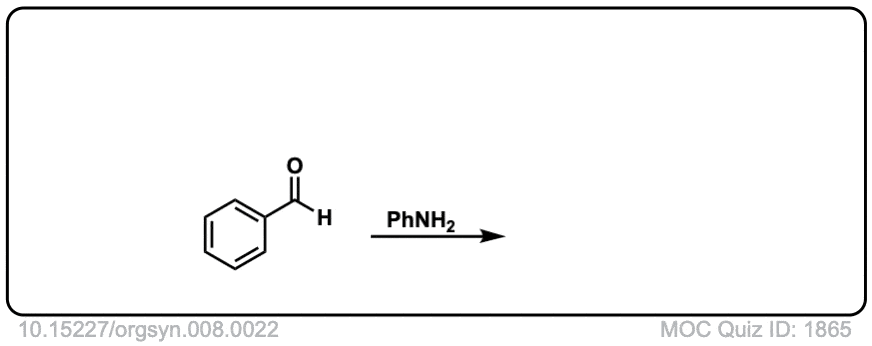
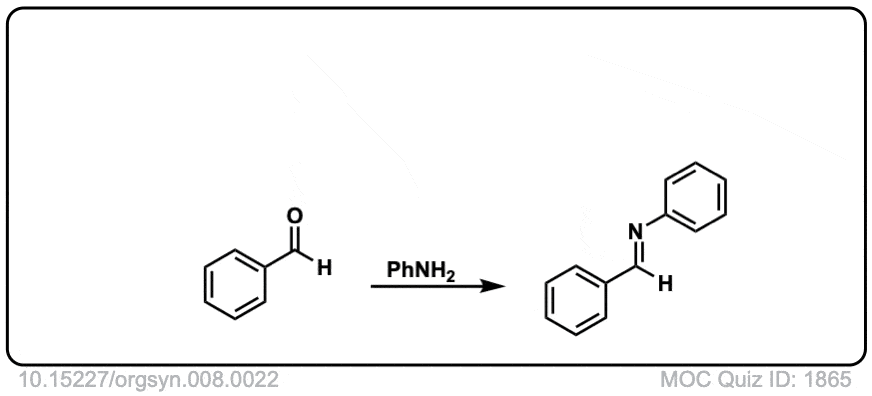
Org. Synth. 1928, 8, 22
DOI Link: 10.15227/orgsyn.008.0022
 Click to Flip
Click to Flip
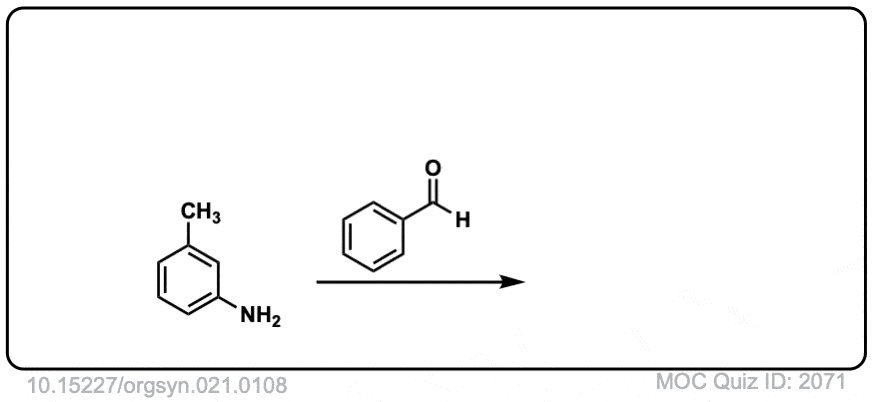
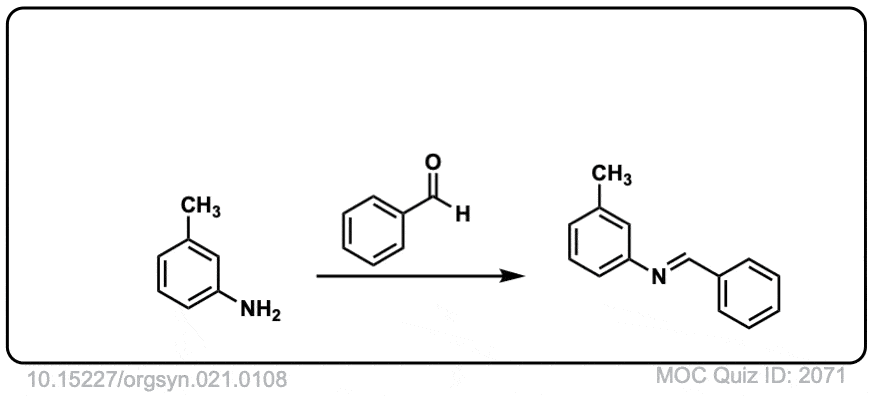
Org. Synth. 1941, 21, 108
DOI Link: 10.15227/orgsyn.021.0108
 Click to Flip
Click to Flip
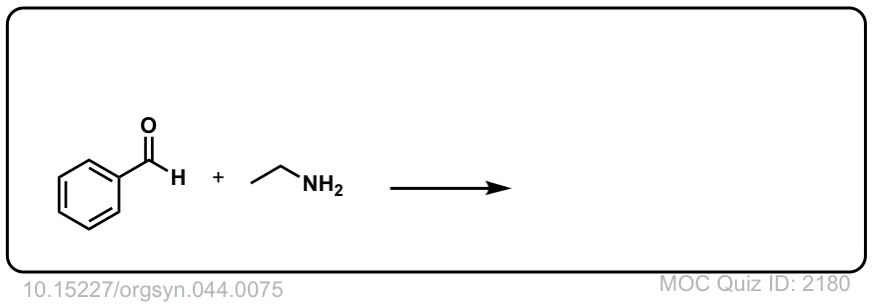
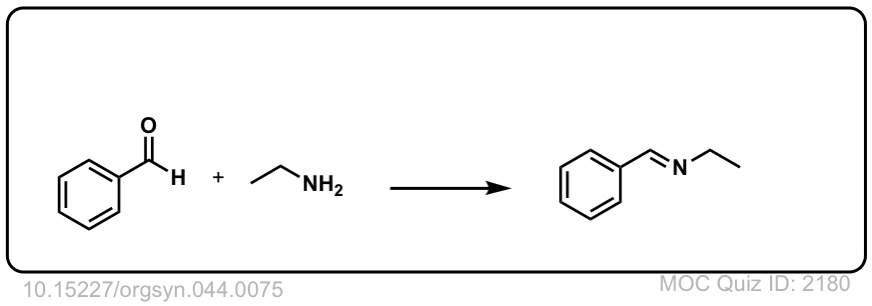
Org. Synth. 1964, 44, 75
DOI Link: 10.15227/orgsyn.044.0075
 Click to Flip
Click to Flip
Hello, thank you for this amazing website! For the second real life example, would there not be two products, one where the methyl group is above the imine and the other where it is below?
Hi! I’m not totally sure what that would look like. The C-N single bond has free rotation so there are other conformational isomers possible, but this should be the only product.
If you sent me a pic of what you were thinking to james@masterorganicchemistry.com I’ll get right back to you
I have a membership and for some reason it is not letting me view this reaction on the reaction guide?
if we take NH3 instead of R-NH2 than what changes occur here
You’d form the imine R2C=N-H. Not something you’d want to isolate, but would be a useful intermediate if you wanted to form an amine through reductive amination.
why protonation not take place at amine instead of carbonyl oxygen even nitrogen have less EN than oxygen.
I think you need to revisit the factors which govern nucleophilicity. Having a lower EN than oxygen means that nitrogen will be more nucleophilic. https://www.masterorganicchemistry.com/2012/06/18/what-makes-a-good-nucleophile/
why imine is most stable then enamine
Do you have a specific example you’d like to refer to? Otherwise I’m not 100% certain we’d be discussing the same thing.
Is this the same mechanism for the 2,4-dinitrophenylhydrazine (2,4-DNP) and ketones?
Yes it is!!
My textbook shows this reaction in water. Without acid, is the carbinolamine a stable form?
Imine formation IN water – or is it imine hydrolysis?
Imine formation in water is not going to be successful unless you are forming a particularly stable imine (such as a cyclic 5 or 6 membered imine) and even then you’ll probably get a lot of the carbinolamine.
For the first step, why does e proton source attack the carbonyl oxygen instead of the more basic amine in the reaction mixture? Is it because of resonance in e protonated carbonyl?
Do we add e acid and e amine at the same time?
Can the acid be a weak acid as strong acidic conditions may protonate e amine and render it non nucleophilic?
Thanks :)
I was wondering this as well.
The amine is more basic, this is true! Acid can attack the amine, but it doesn’t go anywhere (the amine and protonated amine are in equilibrium). It’s a dead end, in other words.
You just need a little bit of protonated carbonyl to get the reaction going.
It’s important that the solution not be too acidic, since if all the amine is protonated (irreversibly) then there is no nucleophile that would be able to attack the carbonyl.
Why would protonation of the amine be irreversible?
Very well written and illustrated articles – many thanks.
JT
why primary amines under diazonium reaction
If you use secondary amines you can’t form the triple bond.