Acidic Hydrolysis of Nitriles To Amides
Description: Nitriles can be hydrolyzed with aqueous acid to give primary amides.
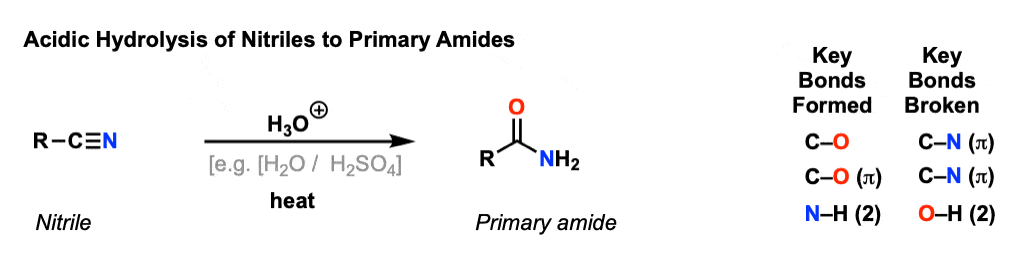
Notes: The acid can be HCl.
See Also: Hydrolysis of nitriles to give carboxylic acids [link].
Examples:
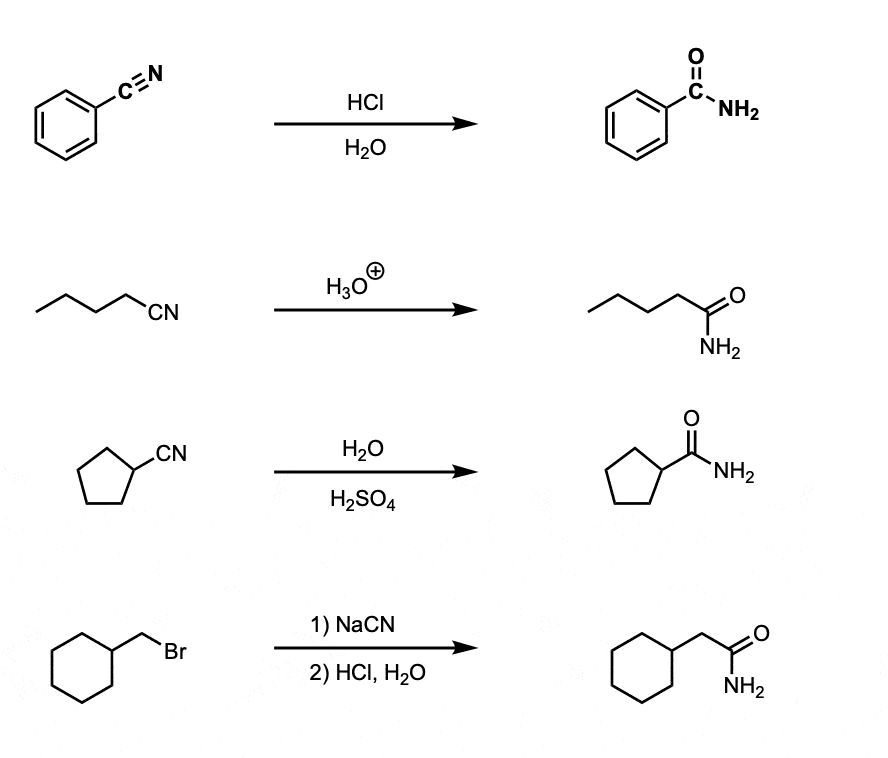
Notes:
The acid is commonly HCl or H2SO4, or alternatively just written “H3O+” to avoid specifics.
Note that example 4 is a nucleophilic substitution (SN2) with NaCN followed by hydrolysis to get the amide.
Mechanism:
The first step of this mechanism is protonation of the amide nitrogen with strong acid (Step 1, arrows A and B). This activates the nitrile carbon towards nucleophilic attack, which occurs with water (Step 2, arrows C and D). The oxygen is then deprotonated (Step 3, arrows E and F).
The next steps (proton transfer and tautomerization) can happen in any order. Here, we start with protonation of the nitrogen (Step 4, arrows G and H) followed by tautomerization, where a C-O pi bond forms and the C-N pi bond breaks (Step 5, arrows I and J). Note that it’s common to show protonation occurring at the same time as tautomerization but we’ve split it into two separate steps here.
The final step is deprotonation (Step 6, arrows K and L) to give the neutral primary amide.
So to summarize, this is a pretty standard PADPED mechanism with a tautomerization step thrown in the middle (Step 5).
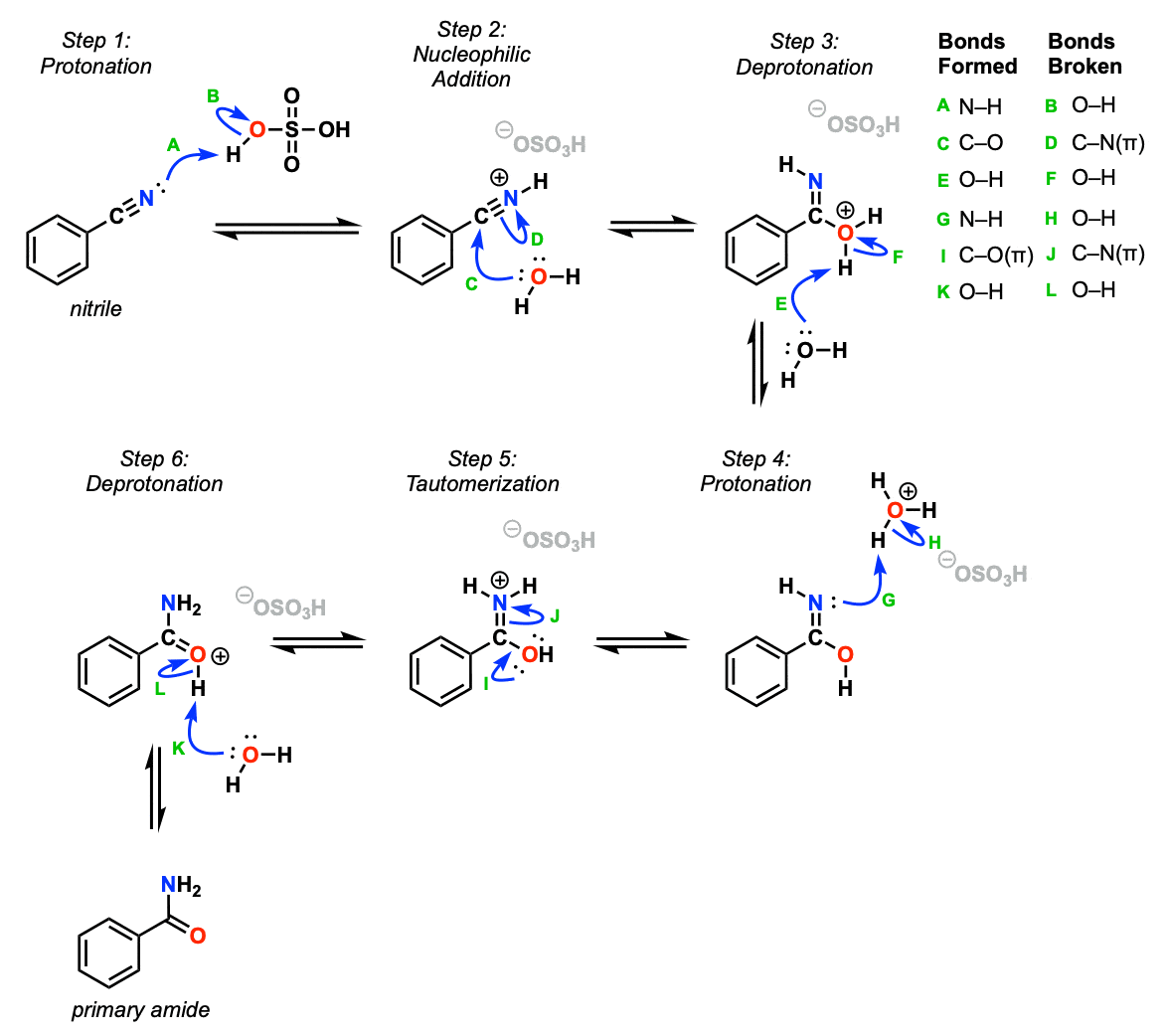
Notes: As noted, it’s common to draw protonation occurring at the same time as tautomerization. Here it’s been noted as a separate step so it doesn’t seem as magical.
(Advanced) References and Further Reading