Free Radical Bromination of Alkanes
Description: When treated with bromine (Br2) and light (hν) alkanes are converted into alkyl bromides. In the absence of any double bonds, with Br2 this is selective for tertiary carbons.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
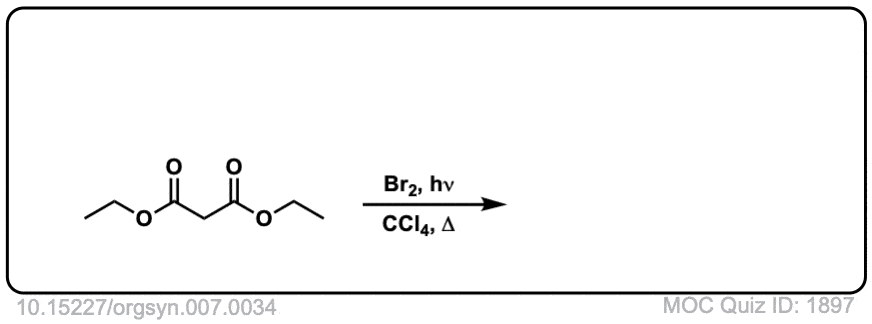
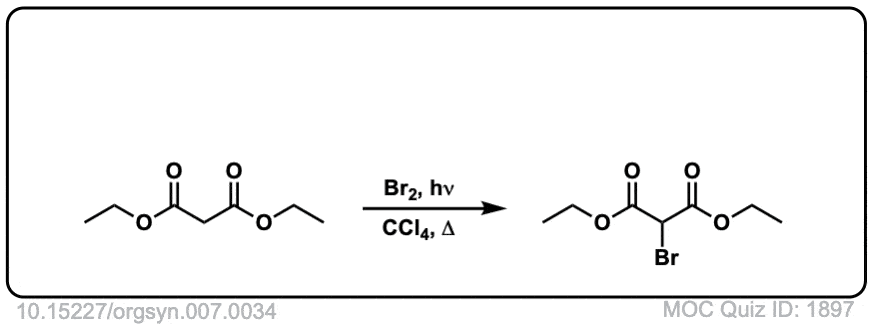
Org. Synth. 1927, 7, 34
DOI Link: 10.15227/orgsyn.007.0034
 Click to Flip
Click to Flip
“At any given time only a small amount of these radicals is present” is stated, but the arrows show that the reaction favors the product of Bromine radicals. is this a mistake?
Thanks for noticing. Fixed the image so that it properly shows Br2 as major and Br radicals as minor.
The narratives on this site are great. I wonder about something. Is there a factor that prevents radical bromination at several carbons in an alkane? I understand that the major products that result from bromination or pentane are the mono-Bromo products, 2-bromo and 3-bromo with miniscule probability of 1-Bromo due to selectivity toward bromination of the secondary carbons of pentane. But what about di-bromo and even tri-bromo products? Where does 2-3-4 tribromo-pentane lie on the selectivity spectrum? Or is there something obvious I missed that precludes di and tri products?
Sorry i just saw the explanation but I still don’t understand why the relative weakness of H-Br-Bonds is causal for the tertiary selectivity.
Using Hess’ law you can calculate delta H for the reaction. Breaking bonds has an energy cost, formation of bonds has an energy gain. The “gain” in energy from forming H-Br is small relative to H-Cl and H-F. Therefore delta H will be less negative for the reaction. Therefore only weak C-H bonds like tertiary and allylic will participate due to these unfavorable thermodynamic properties.
So is there an explanation why Bromine prefers tertiary carbons while Chlorine rather takes primary or secondary ones?
Yes, you can also see this post. https://www.masterorganicchemistry.com/2013/10/31/selectivity-in-free-radical-reactions-bromine-vs-chlorine/
Shouldn’t a C2-Br bond be formed and a Br-Br bond broken?
Fixed
Is there a mistake in Step 3? You listed that in arrow D, a Br-Br bond is formed, and in arrow E, a C2-Br bond is broken. Shouldn’t it be the reverse?
Shoot. You are correct. Will fix.
Hi, there is table with comparison of selectivity. For Br-: 0,007; 1; 220; 19,4. You write that Br- “likes” tertiary carbon. Why there is not the highest number? Thx, P.
Did you mean to say “19,400 ” ?
Sorry, I can see 19 and there is “comma”, not “dot” —> 19 400. It is clear for me now.
what about chain termination?
Chain termination can be drawn by showing ANY of the radicals depicted here combining with the carbon radical. Any time you have two radicals combining to form a new bond, that is chain termination.
this may seem silly, but i just cant remember–are rearrangements possible in radical bromination?
If you’re asking if H and alkyl shifts are possible, for our purposes the answer is no.
Dear Sir,
I see that exciting topic. But you give me some example for “heat may also suffice”. What difference hv and heat are inside this reaction ?
“Notes: Although light (hν) is commonly employed as the initiator, heat may also suffice.”
thanks
Thanks for asking for clarification – what I should have said is that one can also use heat and peroxides. A common example of a peroxide for these types of reactions is tert-butyl peroxide, (CH3)3C–O–O–C(CH3)3 . When this is heated, it fragments to give the free radical (CH3)3CO• , which can fragment Br–Br homolytically to give Br• and start the chain reaction.