Nitration of aromatic groups
Description: Aromatic groups can be nitrated by the action of nitric acid and an acid catalyst
This page is available to MOC Members only.
Sign up here for about 30 cents/ day!
Real-Life Examples:
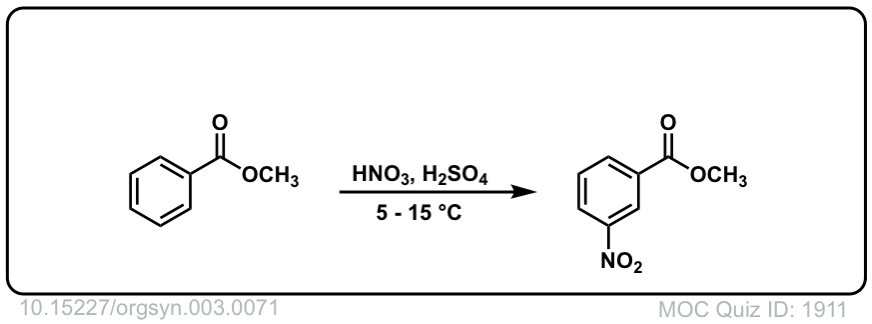
Org. Synth. 1923, 3, 71
DOI Link: 10.15227/orgsyn.003.0071
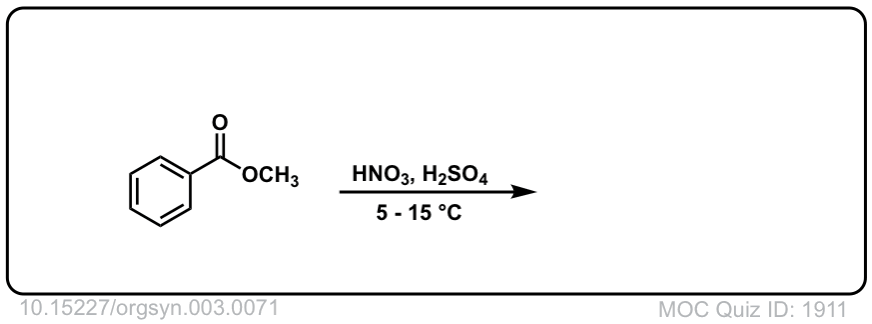 Click to Flip
Click to Flip
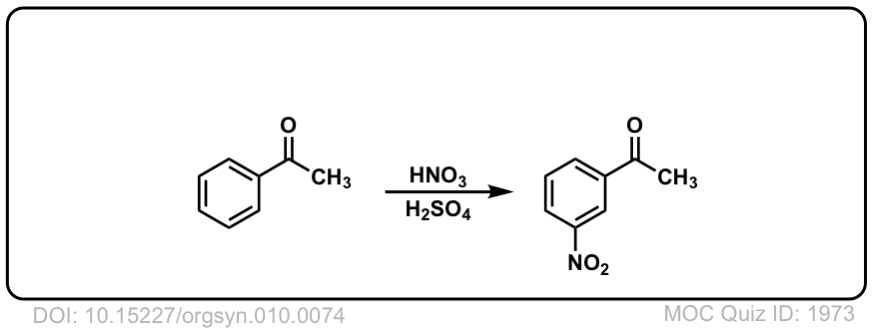
Org. Synth. 1930, 10, 74
DOI Link: 10.15227/orgsyn.010.0074
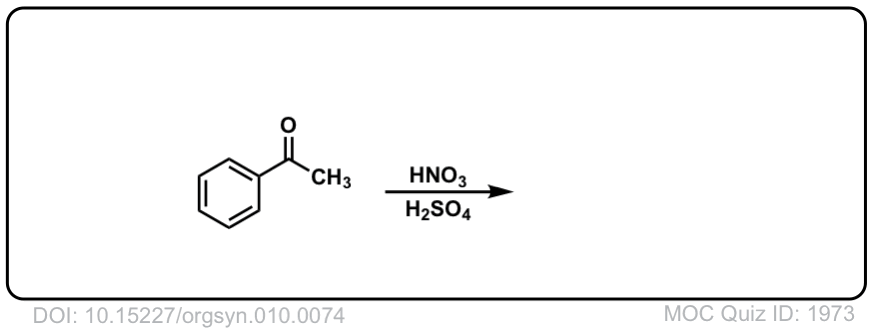 Click to Flip
Click to Flip
Org. Synth. 1934, 14, 68
DOI Link: 10.15227/orgsyn.014.0068
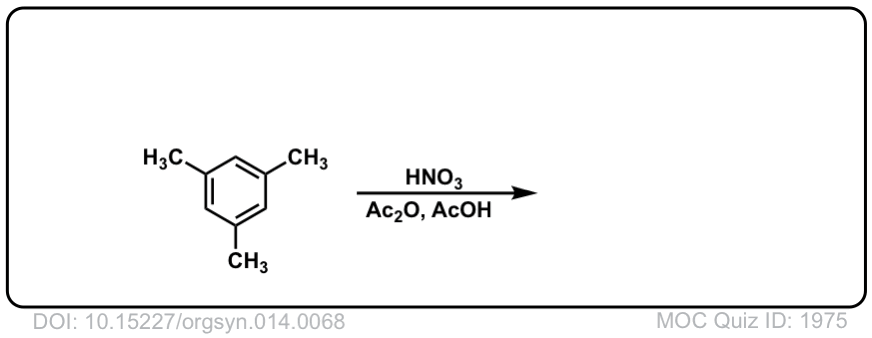 Click to Flip
Click to Flip
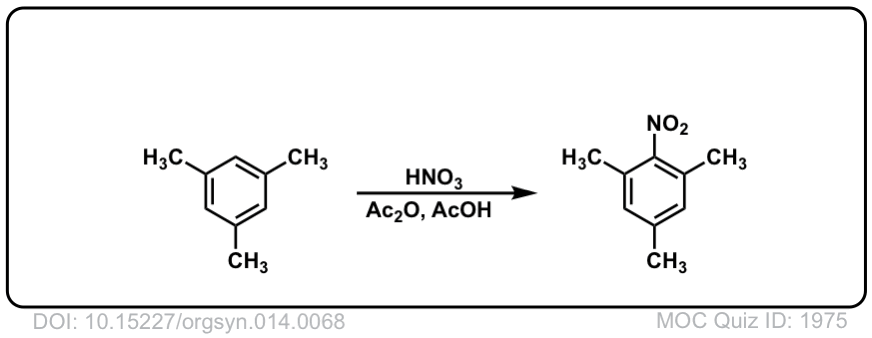
Org. Synth. 1942, 22, 48
DOI Link: 10.15227/orgsyn.022.0048
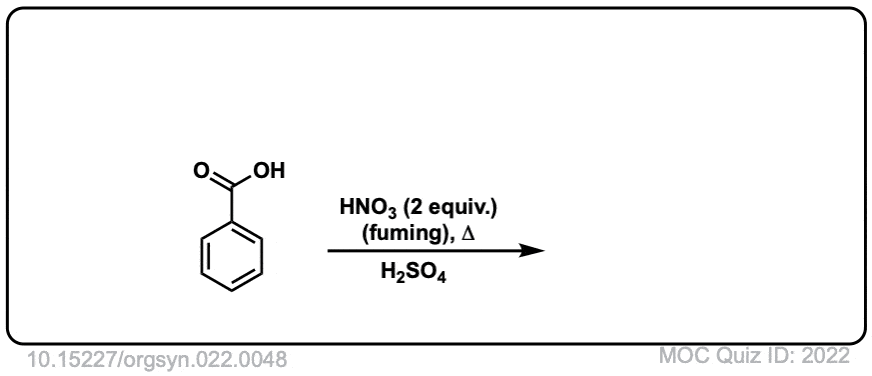 Click to Flip
Click to Flip
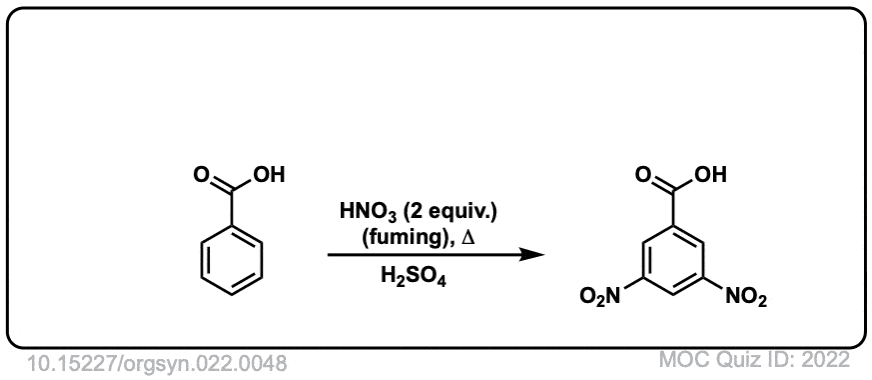
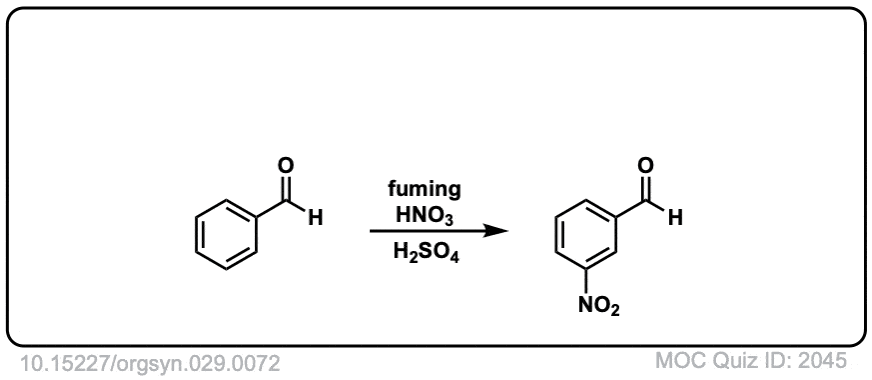
Org. Synth. 1949, 29, 72
DOI Link: 10.15227/orgsyn.029.0072
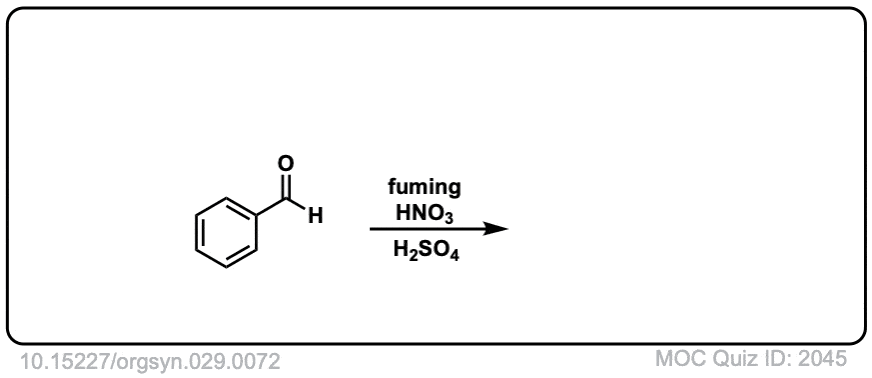 Click to Flip
Click to Flip
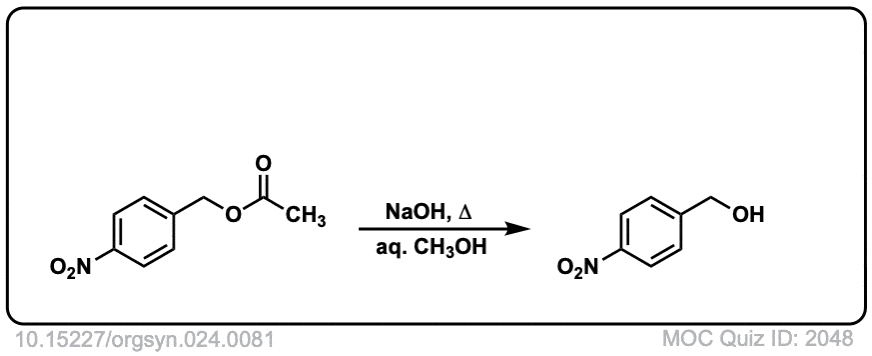
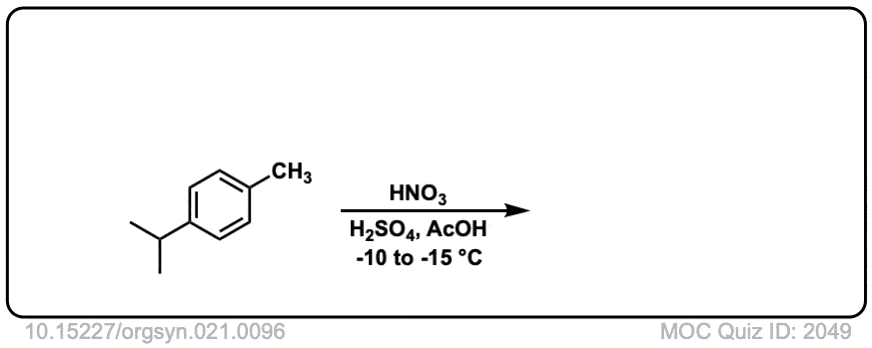
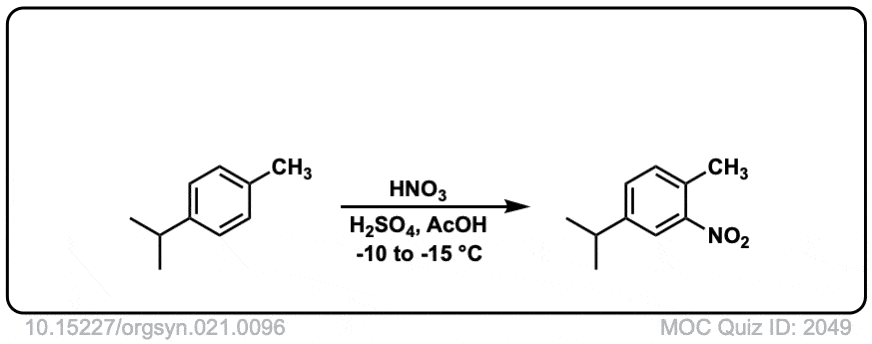
Org. Synth. 1941, 21, 96
DOI Link: 10.15227/orgsyn.021.0096
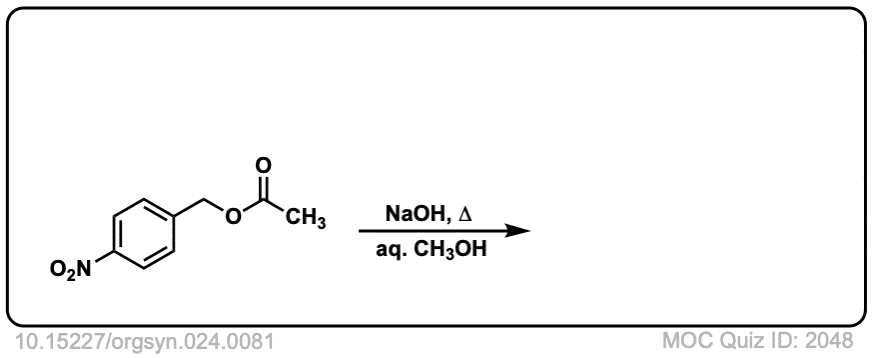 Click to Flip
Click to Flip
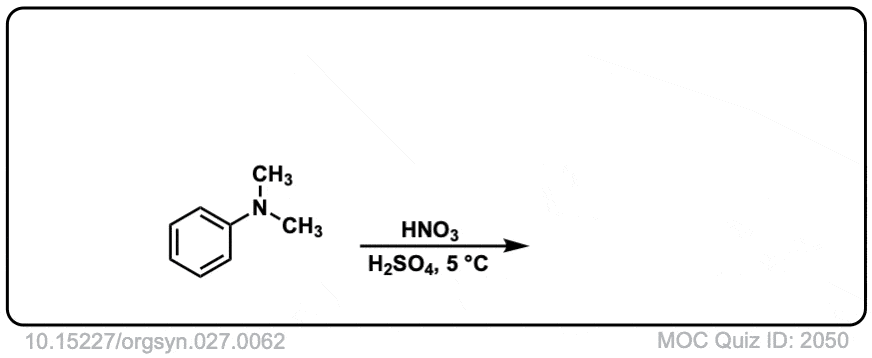
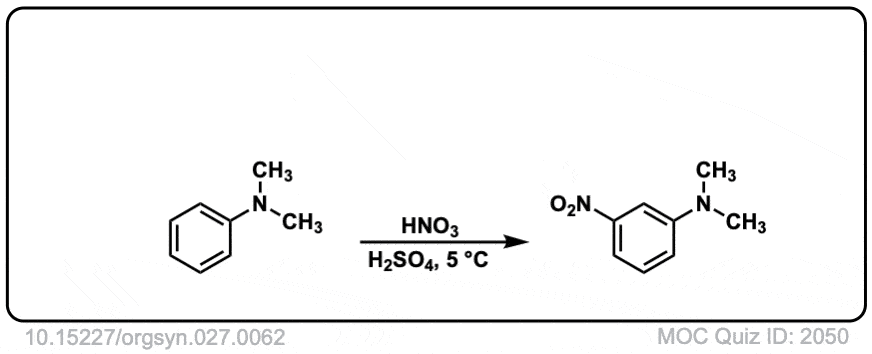
Org. Synth. 1947, 27, 62
DOI Link: 10.15227/orgsyn.027.0062
 Click to Flip
Click to Flip
Org. Synth. 1945, 25, 78
DOI Link: 10.15227/orgsyn.025.0078
 Click to Flip
Click to Flip
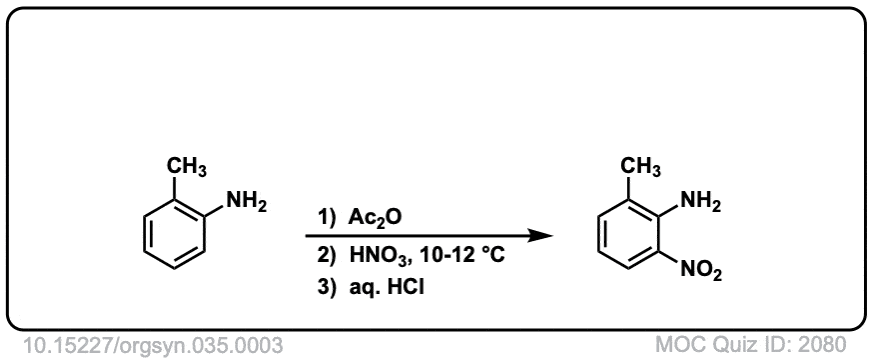
Org. Synth. 1955, 35, 3
DOI Link: 10.15227/orgsyn.035.0003
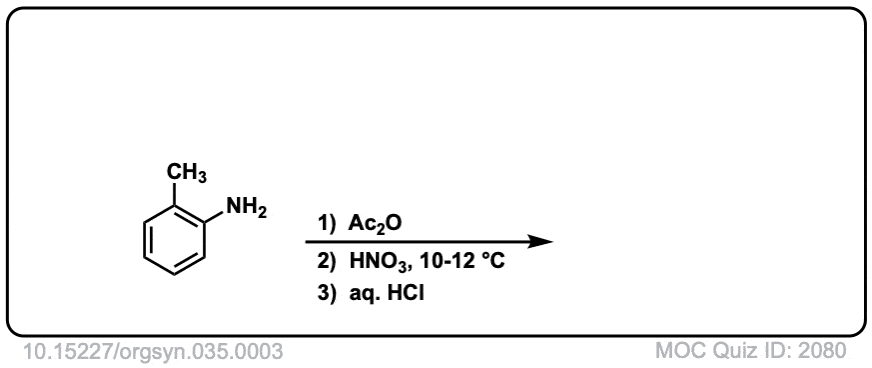 Click to Flip
Click to Flip
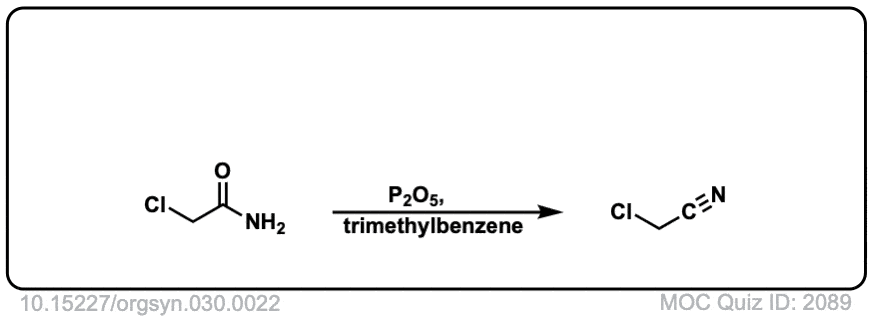
Org. Synth. 1950, 30, 22
DOI Link: 10.15227/orgsyn.030.0022
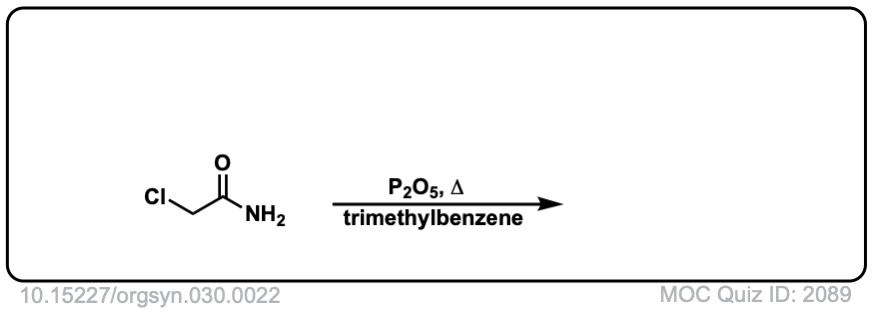 Click to Flip
Click to Flip
Why does the real-life example [Org. Synth. 1945, 25, 78] shows meta substitution, even though -NR2 is an activating group
That is a great question.
This is performed in strongly acidic conditions. Under these conditions the NMe2 is protonated to give -NMe2H (+) where the nitrogen no longer has a lone pair. Since there is no lone pair available it can no longer act as a pi-donor, and since nitrogen is electronegative relative to carbon, it behaves as an electron-withdrawing group.
So the result is that it behaves as a meta director under acidic conditions!
Hessung, the reason is because it is a Lewis acid base reaction between the sulfuric acid and the nitrate ion. This is a necessary step in preparing the two groups for the SeaR reaction to follow.
For step 1, the half arrow leading to OH2 should be a full arrow?
It’s meant to be a full arrow, new diagrams will be up soon that make it much more clear.
In the mechanism , on step one, why the oxygen which has negative charge attack hydrogen first?
why are there two lone pairs on the top oxygen in the middle figure between step one and two of the mechanism? wasn’t one of the pairs used to grab the additional hydrogen?
Oops. Typo – thanks for pointing it out!