Oxidative cleavage of 1,2-diols to give aldehydes/ketones
Description: Treatment of vicinal diols (1,2-diols) with an oxidant such as NaIO4, HIO4, or Pb(OAc)4 cleaves C–C bonds and forms carbonyl compounds.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
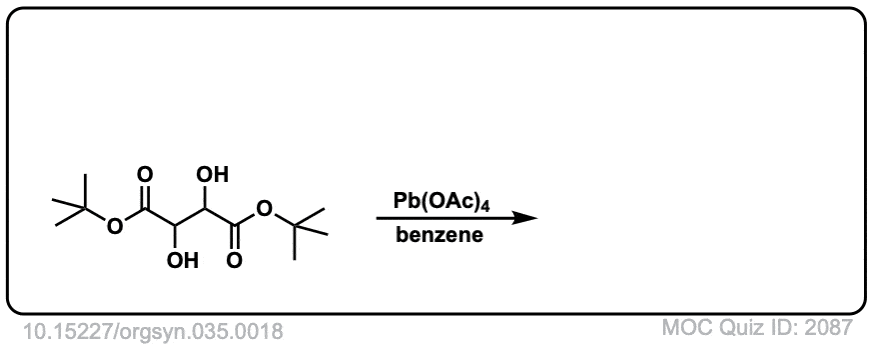
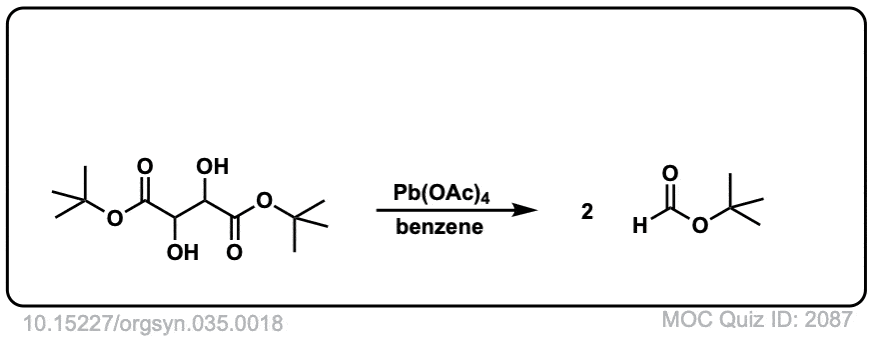
Org. Synth. 1955, 35, 18
DOI Link: 10.15227/orgsyn.035.0018
 Click to Flip
Click to Flip
When I did Oxydative Clivage of protected D-Manitol (1-2 & 5-6 OH is Protected) with NaIO4, than I got issue of Yield, (I took 10 gm of protected D-Manitol than yield was very less) and I also got an issue to reduce this aldyhide into alcholol.
Please suggest me the right way to extract (2,2-dimethyl-1,3-dioxolan-4-yl)methanol from protected D-Monitol
Thanks in Advance !!
I have no doubt this is a well precedented reaction and I would advise you consult the available literature on it.
when does it become a ketone and when does it become an aldehyde
Tertiary alcohols become ketones; secondary alcohols become aldehydes; primary alcohols break off and become formaldehyde (which likely will be oxidized to CO2 under the reaction conditions)
I have was just wondering if compounds, other than lead (IV) acetate, could be used in this oxidative cleavage? Such as Tin (IV) acetate or other acetate salts like ferric acetate or cupric acetate?
Sodium periodate will do it, as will periodic acid. Ruthenium tetraoxide will do this too. Tin acetate is not an oxidant; copper acetate won’t do it; Iron acetate will not lead to this oxidation in the absence of other oxidants. One could maybe add hydrogen peroxide to the Fe(III), but then all hell would break loose.
I was struggled do my uni organic synthesis tutorial . this helped me a lot. thanks
can we have 2 alcohols on same carbon…. ???
Yes, it’s called a “geminal diol”, or sometimes, a “hydrate”. It will not undergo oxidative cleavage however
My question is regarding Lead(IV) acetate. Does the initial compound have to have both a secondary and tertiary alcohol in order to have the reaction or can the reaction occur in the presence of just a tertiary or a secondary alcohol? In other words, could the reaction occur if the initial compound was a simple alkane with a secondary alcohol attached? Thank you! Your website and resources are fantastic and very helpful :D
Hi Crystal – you absolutely need two adjacent alcohols. It won’t work with a single isolated alcohol. Hope this helps – James