The Gabriel synthesis of amines
Description: The Gabriel synthesis is a method for making primary amines through an SN2 reaction of a phthalimide with an alkyl halide followed by cleavage with hydrazine (NH2NH2).
This page is available to MOC Members only.
Sign up here for about 30 cents/ day!
Real-Life Example:
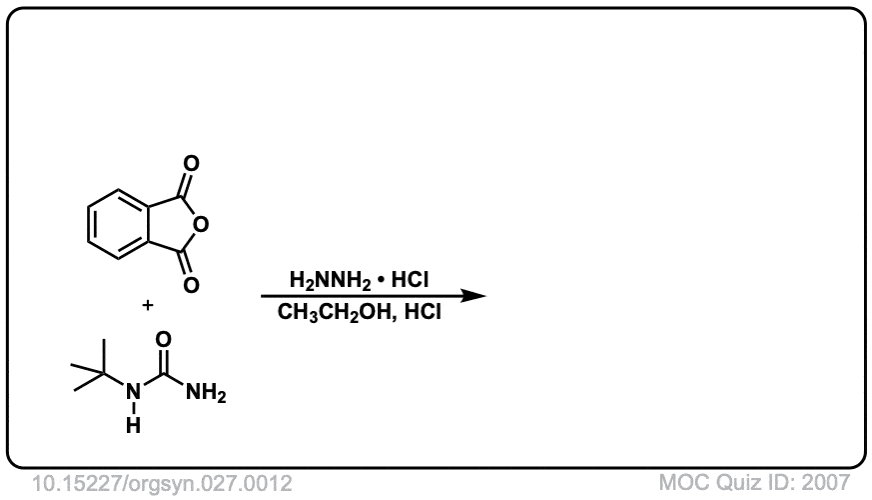
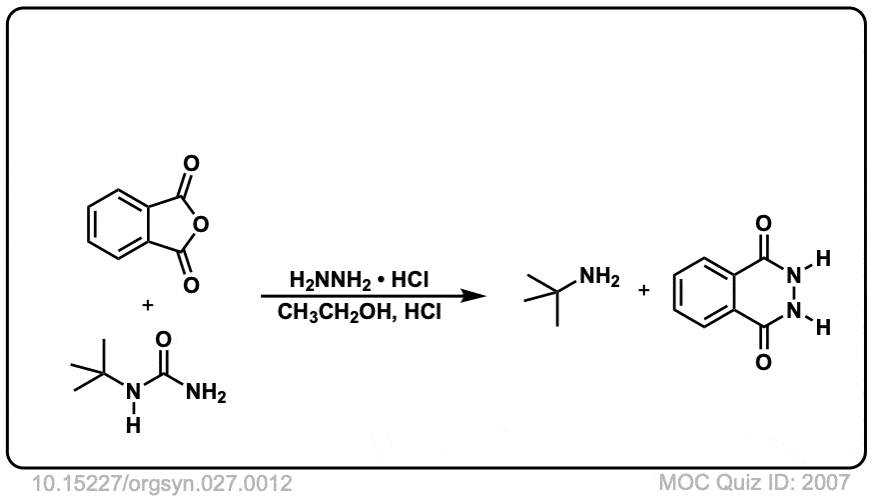
Org. Synth. 1947, 27, 12
DOI Link: 10.15227/orgsyn.027.0012
 Click to Flip
Click to Flip
Hi, in step 6 and 7 you are missing the letter R.
Fixed, thank you!
Hi,
When would you use Gabriel vs Azide to make a primary amine in synthesis?
Great question! For uncomplicated molecules without a lot of different functional groups, they both work.
From a practical standpoint, N3- tends to get more use.
N3- tends to be a better nucleophile (faster rxn) than potassium phthalimide. Also, reduction of N3 to NH2 can be done many different ways (Pd-C/H2, LiAlH4, etc) and only blows off N2, whereas cleavage of the phthalimide necessitates getting rid of the byproduct.
For your purposes though – either one is probably fine!
Hi James,
Which functional groups will not tolerate either/both of these transformations (i.e. azide SN2 & Gabriel rxn)?
Thanks!
That’s kind of a broad question. With the Gabriel you’d want to avoid any unprotected aldehydes/ketones for a variety of reasons, particularly the deprotection step. That’s just a start.